Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25C.
Question:
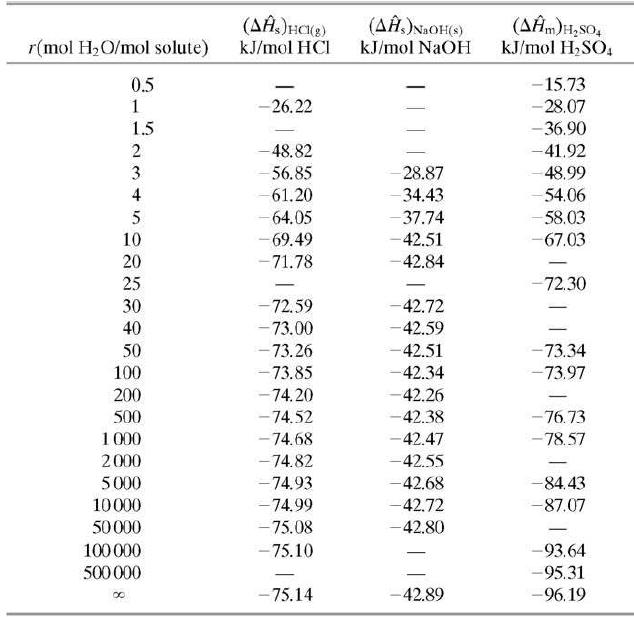
Five cubic meters of a 1.00-molar aqueous sulfuric acid solution (SG = 1:064) is stored at 25°C. Use data in Tables B.1 and B.11 to calculate the standard heat of formation of the solution in kJ/mol H2SO4 relative to the solute elements and water, and the total enthalpy of the solution relative to the same reference conditions.
Table B.11
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Question Posted:





