For monocyclic conjugated polyenes (such as cyclobutadiene and benzene) with each of N carbon atoms contributing an
Question:
For monocyclic conjugated polyenes (such as cyclobutadiene and benzene) with each of N carbon atoms contributing an electron in a 2p orbital, simple Hückel theory gives the following expression for the energies Ek of the resulting π molecular orbitals:
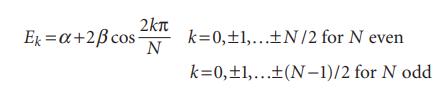
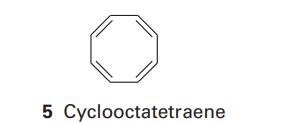
(a) Calculate the energies of the π molecular orbitals of benzene and cyclooctatetraene (5). Comment on the presence or absence of degenerate energy levels.
(b) Calculate and compare the delocalization energies of benzene (using the expression above) and hexatriene. What do you conclude from your results?
(c) Calculate and compare the delocalization energies of cyclooctatetraene and octatetraene. Are your conclusions for this pair of molecules the same as for the pair of molecules investigated in part (b)?
Step by Step Answer:

Physical Chemistry Thermodynamics And Kinetics
ISBN: 9781464124518
10th Edition
Authors: Peter Atkins, Julio De Paula





