For the reaction I (aq) + OCl (aq) OI (aq) + Cl (aq) occurring in aqueous solution,
Question:
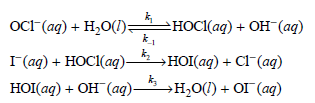
a. Derive the rate law expression for this reaction based on this mechanism.
b. The initial rate of reaction was studied as a function of concentration by Chia and Connick [J. Phys. Chem. 63 (1959), 1518], and the following data were obtained:
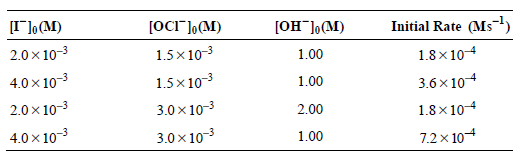
Is the predicted rate law expression derived from the mechanism consistent with these data?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





