From the following data, calculate ÎH o R,391.4 K for the reaction CH 3 COOH(g) + 2O
Question:
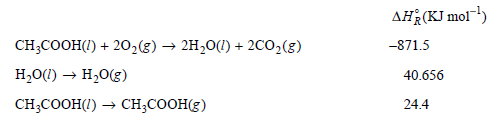
Values for ΔHoR for the first two reactions are at 298.15 K, and for the third reaction at 391.4 K.

Transcribed Image Text:
AH(KJ mol) CH;COOH(I) + 20,(g) → 2H20(1) + 2C0,(g) H,0(1) → H,0(g) CH,CООН() — сH,CОOHE) -871.5 40.656 24.4 Substance CH-COон() 0,(g) co:(g) Н.0() H,0(g) 14.9 3.53 4.46 9.055 4.038 |CP,m/R
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
The desired overall reaction can be related to the individual reactions f...View the full answer

Answered By

Ehsan Mahmood
I’ve earned Masters Degree in Business Studies and specialized in Accounts & Finance. Couple with this, I have earned BS Sociology from renowned institute of Pakistan. Moreover, I have humongous teaching experience at Graduate and Post-graduate level to Business and humanities students along with more than 7 years of teaching experience to my foreign students Online. I’m also professional writer and write for numerous academic journals pertaining to educational institutes periodically.
4.90+
248+ Reviews
287+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the following data, Calculate the enthalpy change for the reaction 2C(graphite) + 3H2(g) - C2H6(g) C(graphite) 02(g)CO2(8) H208)02(8) H0U) 2C2Hs (g)702(g)4CO2(g) 6H2O(l) AHn393.5 kJ/mol...
-
From the following data for the first-order gas-phase isomerization of CH3NC at 215 oC, calculate the first-order rate constant and half-life for the reaction: Time (s) Pressure CH3NC (torr)...
-
Rate constants for a reaction were determined at five temperatures. From the following data, calculate the experimental energy of activation and then calculate G¡, H¡, and S¡ for...
-
In schema normalisation, is Boyce-Codd Normal Form (BCNF) always to be preferred over 3rd Normal Form (4NF)? Explain your answer. [5 marks] 8 (TURN OVER) CST.2004.7.4 8 Economics, Law and Ethics (a)...
-
Thompson Company uses a standard cost system for its single product. The following data are available: Actual experience for the current year: Purchases of raw materials (11,000 yards at $11.00 per...
-
Josh is climbing up a steep \(34^{\circ}\) slope, moving at a steady \(0.75 \mathrm{~m} / \mathrm{s}\) along the ground. How many meters of elevation does he gain in one minute of this climb?
-
Discuss under what circumstances parental consent for a minor might not be necessary.
-
Vail Resorts, Inc., owns and operates five premier year-round ski resort properties (Vail Mountain, Beaver Creek Resort, Breckenridge Mountain, and Keystone Resort, all located in the Colorado Rocky...
-
Sarif is interested in an alternative investment strategy fund with an investment mandate focused on anticipating movements in the market prices of commodities. What type of strategy would this type...
-
A young woman named Seema (22) succumbed to injuries at a private hospital inGuwahati on 1st June, 2013 due to multiple organ failure as she had developedsevere health issues due to swallowing acid....
-
Predict the major product obtained upon radical bromination of each of the following compounds: (a) (b) (c)
-
A cylindrical vessel with rigid adiabatic walls is separated into two parts by a frictionless adiabatic piston. Each part contains 45.0 L of an ideal monatomic gas with C V ,m = 3/2R. Initially, T i...
-
Define the following terms: (a) Slope (b) y-intercept
-
Which one of the following lists only includes amounts that are components of earned income for RRSP purposes Automobile standby charge salespersons expenses and resource royalties Business income or...
-
If a corporation app Question Content Area On January 1, 2020, Medley Corporation sold $200,000 of its 14%, five-year bonds dated January 1, 2020, for $206,000 total cash. The bonds sold appropriated...
-
Solve the equation using logarithms, 9* -10 1
-
How does the commodification of ethnic art impact the autonomy and agency of indigenous artists and communities, and what measures can be taken to address these concerns ?
-
Determine the second derivative of 2x + y = 1 4y[Hint: Implicit Differentiation]
-
What is the origin of most of the natural radiation we encounter?
-
Open Text Corporation provides a suite of business information software products. Exhibit 10-9 contains Note 10 from the companys 2013 annual report detailing long-term debt. Required: a. Open Text...
-
Some of the selection rules for hydrogenic atoms were derived in justification 10.4. Complete the derivation by considering the x- and y components of the electric dipole moment operator.
-
The wave function of a many-electron closed-shell atom can expressed as a Slater determinant (Section 10Ab). A useful property of determinants is that interchanging any two rows or columns changes...
-
The distribution of isotopes of an element may yield clues about the nuclear reactions that occur in the interior of a star. Show that it is possible to use spectroscopy to confirm the presence of...
-
Question 5 In 50 words or more, explain how advertising is implemented through each type of media listed below. Advertising Media Types How Advertising is Implemented Through Each Type of Media i....
-
1. Research and outline the Lean Method and how it applies to marketing a products. Explain in one fifty words. 2. Outline your understanding of market research and what is should include: Be...
-
MUN Faculty of Business Administration | Managing Social Enterprises: Marketing Ultimate project goal Write a letter that appeals to me (or your audience), and convinces them to take a key action, or...

Study smarter with the SolutionInn App


