From the following data, derive the absolute entropy of crystalline glycine at T = 300.K. You can
Question:
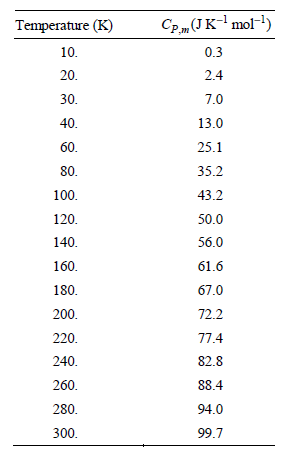
Transcribed Image Text:
CP„(JK-mol-) Temperature (K) 10. 0.3 20. 2.4 30. 7.0 13.0 40. 60. 25.1 35.2 80. 100. 43.2 50.0 120. 140. 56.0 160. 61.6 180. 67.0 72.2 200. 220. 77.4 82.8 240. 260. 88.4 280. 94.0 99.7 300.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
The line in the graph above of C Pm vertical axis against T is the best fit to the ...View the full answer

Answered By

Pranav Makode
I am a bachelor students studying at professor ram meghe institute of technology and research. I have a great experience of being an expert. I have worked as an expert at helloexperts and solvelancer as a part time job. I have also worked as a doubt solver at ICAD SCHOOL OF LEARNING, which is in Amravati city. I have also worked as an Freelancer.
I have great experience of helping students, as described above. I can help any students in a most simple and understandable way. I will not give you have any chance for complaint. You will be greatfull to accept me as an expert.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the following data, determine f Ho for diborane, B2H6 (g), at 298 K: (I) B2H6 (g) + 3 O2 (g) B2O3(s) + 3 H20 (g) t Ho=-1941 kJ mol-1 (2) 2 B(s) + t Oh) B2O3(S) t, Ho = -2368 kJ mol-1 (3) H2 (g)...
-
From the following data for liquid nitric acid, determine its heat of vaporization and normal boiling point. Temperature C) Vapor Pressure (mm Hg) 10. 20. 30. 40. 50. 80. 14.4 26.6 47.9 81.3 133 208...
-
From the following data for three prospective fuels, calculate which could provide the most energy per unit volume: Density at 20 C Molar Enthalpy of Combustion Fuel (g/cm (kJ/mol) Nitroethane, C2H...
-
Understand the content theories of motivation.
-
Why are slow-moving items dangerous to the small business? What can be done to liquidate them from inventory?
-
Describe how bases interact with each other in the double helix. This description should include the concepts of complementarity, hydrogen bonding, and base stacking.
-
Derive the solutions for transient concentration profiles in the two-bulb apparatus (Example 21.5 in the text) for the binary case, and show that the multicomponent case can be derived as an...
-
Kopecky Industries Inc. is considering allocating a limited amount of capital investment funds among four proposals. The amount of proposed investment, estimated income from operations, and net cash...
-
2) What is the negation of this proposition? [0.5pt] "If it is below freezing, it is snowing" a) It is not below freezing, and it is not snowing b) It is below freezing, and it is not snowing c) It...
-
Elk County Telephone has paid the dividends shown in the following table over the past 6 years. Year Dividend per share 2012 .......$2.87 2011 ....... 2.76 2010 ....... 2.60 2009 ....... 2.46 2008...
-
How many finished units per day can the current assembly process produce? Where are the bottlenecks?
-
If the process needed to make 18 units per day, what should you do?
-
A quantity of 7.480 g of an organic compound is dissolved in water to make 300.0 mL of solution. The solution has an osmotic pressure of 1.43 atm at 27C. The analysis of this compound shows that it...
-
The system has a transfer function \(\mathrm{P}(\mathrm{s})=\frac{2}{s+2}\). The gain for \(\omega=2 \mathrm{rad} / \mathrm{sec}\) will be (a) 0.707 (b) 0.666 (c) 0.5 (d) 0.25
-
Using letters A, T, E for the component statements, translate the following compound statements into symbolic notation. a. If Anita wins the election, then tax rates will be reduced. b. Tax rates...
-
Suppose you see a car with an advertised price of \(\$ 18,490\) at \(\$ 480\) per month for 5 years. What is the amount of interest paid?
-
Rewrite each of the following statements in the form "If \(A\), then \(B\)." a. Candidate Lu winning the election will be a sufficient condition for property taxes to increase. b. The user clicks...
-
Which one of the following input-output relationship is that of a linear system 1. 3. Output 5 0 Output 0 0 Input Input 2. 4. Output Output 0 0 Input Input
-
Find the average rate of change of f from 0 to /6. f(x) = tan x
-
The first national bank pays a 4% interest rate compound continuously. The effective annual rate paid by the bank is __________. a. 4.16% b. 4.20% c. 4.08% d. 4.12%
-
Solve for the hydrogen-ion concentration in solutions of acetic acid with stoichiometric molarities equal to 0.00100 mol l 1 . Use the method of successive approximations.
-
Verify the prediction of the ideal gas equation of state given in the previous example.
-
Substitute the value of the molar volume obtained in the previous example and the given temperature into the Dieterici equation of state to calculate the pressure. Compare the calculated pressure...
-
1) Based on the stock chart for Michaels Companies Inc, what do you think the short and long-term growth potentials are for this company? (discuss the advantages/disadvantages) Link to the stock...
-
After being drafted in the first round of the NFL draft, a star defensive end invests his signing bonus of $9,827,000.00 in a mutual fund. The fund pays on average 7.00% APR. The player will not...
-
XYZ Co has plans to issue 7,000, Eleven percent Debentures of Rs.100 each at a discount of 5%. The debentures are redeemable after 4 years and the commission payable to brokers & underwriters is Rs....

Study smarter with the SolutionInn App


