Fuel cells have been proposed as an alternative energy technology for use in stationary and transportation applications.
Question:
Fuel cells have been proposed as an alternative energy technology for use in stationary and transportation applications. A fuel cell is an electrochemical device in which hydrogen reacts with oxygen from the air to produce water and DC electricity. The most flexible fuel cell design is the proton exchange membrane fuel cell (PEMFC). A 1-W PEMFC could be used for portable applications such as cellular telephones, and a 100-kW PEMFC could be used to power an automobile.
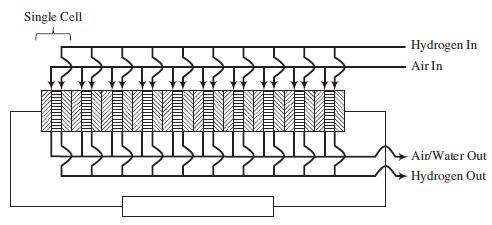
A schematic of a 10-cell stack of fuel cells connected in series is shown on the next page. Each cell consists of an anode (left block with lines sloping up and to the right), electrolyte membrane (center block with horizontal lines), and cathode (right block with line sloping down and to the right). The hydrogen and air are fed in parallel to each cell. Also, the exiting gas flows are collected in parallel.
The following reactions occur inside the PEMFC:
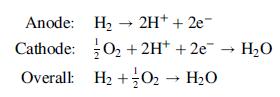
In this problem, we will analyze the flows of chemical species into and out of a PEMFC that could be used to produce electricity for an apartment complex. At full load, the fuel cell operates at a voltage of 26 volts and a current of 500 amps and has a maximum power of 13 kW. A single cell operates at 0.7 V.
(a) According to the U.S. Energy Information Administration (www.eia.doe.gov), a typical household uses 936kW h in a month. Determine the average power usage (kW) per household and use the result to estimate the number of apartment units that could be powered with the 13kW fuel cell.
(b) The hydrogen requirement for a PEMFC is given by (nH2)stoich = IN/2F, where I is the current in A, N is the number of cells, and F is Faraday’s constant, 96,485 coulombs of charge per mol of electrons. Note that in this expression, we use the constant 2 mol electrons per mol of fuel, which follows from the reaction at the anode. If the hydrogen is fed in 15% excess of this amount and the air is fed in 200% excess of the amount required to consume all the hydrogen, what are the flow rates of hydrogen and air required in mol/s and SLPM (standard liters per minute)?
(c) Determine the molar flow rate of hydrogen exiting from the anode and the molar composition of the cathode exit gas.
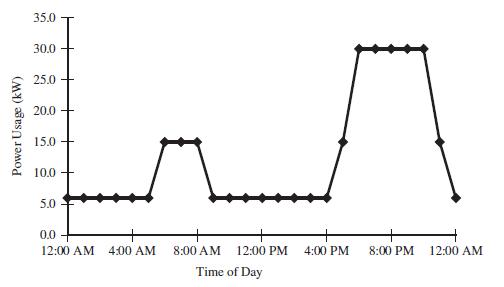
(d) The total electrical power demand in the 10 apartments is given in the plot below. Noting that fuel cells respond quickly to load changes but cannot exceed their rated capacity, calculate how many apartments could be safely powered with a 13kW fuel cell? What is a disadvantage of this practice in terms of power usage?
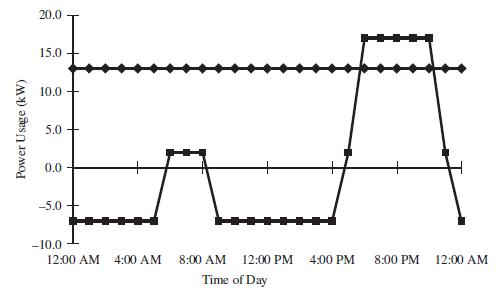
(e) It has been suggested that a hybrid system with a fuel cell and a deep cycle battery should be used. The system works as follows:
• If the power demand exceeds the capacity of the fuel cell, the battery will be used to supply short-term power.
• If the power demand is below the fuel-cell capacity, the battery will be charged by the fuel cell.
The plot below shows the power usage from the fuel cell (diamonds) and the battery (squares) for the same power usage as the figure above. Determine the times when the battery is being charged and the minimum total energy capacity of the battery in kW hr.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




