Fuel oils contain primarily organic compounds and sulfur. The molar composition of the organic fraction of a
Question:
Fuel oils contain primarily organic compounds and sulfur. The molar composition of the organic fraction of a fuel oil may be represented by the formula CpHqOr ; the mass fraction of sulfur in the fuel is xS (kg S/kg fuel); and the percentage excess air, Pxs , is defined in terms of the theoretical air required to burn only the carbon and hydrogen in the fuel.
(a) For a certain high-sulfur No. 6 fuel oil, p = 0.71, q = 1.1, r = 0.003, and xS = 0.02. Calculate the composition of the stack gas on a dry basis if this fuel is burned with 18% excess air, assuming complete combustion of the fuel to form CO2, SO2, and H2O and expressing the SO2 fraction as ppm (mol SO2/106 mol dry gas).
(b) Create a spreadsheet to calculate the mole fractions of the stack gas components on a dry basis for specified values of p, q, r, xS, and Pxs. The output should appear as follows:
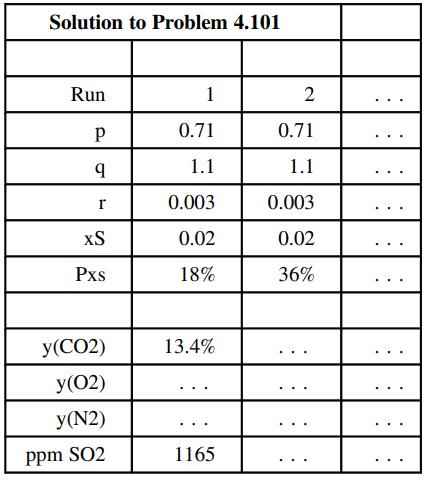
(Rows below the last one shown can be used to calculate intermediate quantities.) Execute enough runs (including the two shown above) to determine the effect on the stack gas composition of each of the five input parameters. Then for the values of p, q, r, and xS given in Part (a), find the minimum percentage excess air needed to keep the dry-basis SO2 composition below 700 ppm. (Make this the last run in the output table.) You should find that for a given fuel oil composition, increasing the percentage excess air decreases the SO2 concentration in the stack gas. Explain why this should be the case.
(c) Someone has proposed using the relationship between Pxs and ppm SO2 as the basis of a pollution control strategy. The idea is to determine the minimum acceptable concentration of SO2 in the stack gas, then run with the percentage excess air high enough to achieve this value. Give several reasons why this is a poor idea.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





