Hydrocarbons are generally considered to be nonpolar or weakly polar at best, characterized by dipole moments that
Question:
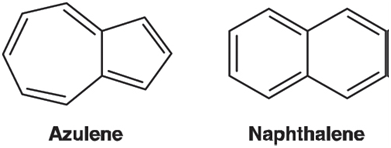
Optimize the geometry of azulene using the HF/6-31G* model and calculate an electrostatic potential map. For reference, perform the same calculations on naphthalene, a nonpolar isomer of azulene. Display the two electrostatic potential maps side by side and on the same (color) scale. According to its electrostatic potential map, is one ring in azulene more negative (relative to naphthalene as a standard) and one ring more positive? If so, which is which? Is this result consistent with the direction of the dipole moment in azulene? Rationalize your result.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





