Methanol is formed from carbon monoxide and hydrogen in the gas-phase reaction The mole fractions of the
Question:
Methanol is formed from carbon monoxide and hydrogen in the gas-phase reaction
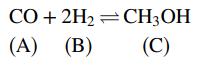 The mole fractions of the reactive species at equilibrium satisfy the relation
The mole fractions of the reactive species at equilibrium satisfy the relation
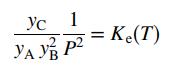
where P is the total pressure (atm), Ke the reaction equilibrium constant (atm-2), and T the temperature (K). The equilibrium constant Ke equals 10.5 at 373 K, and 2.316x10-4 at 573 K. A semilog plot of Ke (logarithmic scale) versus 1/T (rectangular scale) is approximately linear between T = 300 K and T = 600 K.
(a) Derive a formula for Ke(T), and use it to show that Ke(450 K) = 0.0548 atm-2.
(b) Write expressions for nA; nB, and nC (gram-moles of each species), and then yA; yB , and yC, in terms of nA0; nB0; nC0, and ξ, the extent of reaction. Then derive an equation involving only nA0; nB0; nC0, P, T, and ξe , where ξe is the extent of reaction at equilibrium.
(c) Suppose you begin with equimolar quantities of CO and H2 and no CH3OH, and the reaction proceeds to equilibrium at 423 K and 2.00 atm. Calculate the molar composition of the product (yA, yB , and yC ) and the fractional conversion of CO.
(d) The conversion of CO and H2 can be enhanced by removing methanol from the reactor while leaving unreacted CO and H2 in the vessel. Review the equations you derived in solving Part (c) and determine any physical constraints on ξe associated with nA0 = nB0 = 1 mol. Now suppose that 90% of the methanol is removed from the reactor as it is produced; in other words, only 10% of the methanol formed remains in the reactor. Estimate the fractional conversion of CO and the total gram moles of methanol produced in the modified operation.
(e) Repeat Part (d), but now assume that nB0 = 2 mol. Explain the significant increase in fractional conversion of CO.
(f) Write a set of equations for yA; yB ; yC , and f A (the fractional conversion of CO) in terms of yA0; yB0; T, and P (the reactor temperature and pressure at equilibrium). Enter the equations in an equation-solving program. Check the program by running it for the conditions of Part (c), then use it to determine the effects on fA (increase, decrease, or no effect) of separately increasing, (i) the fraction of CH3OH in the feed, (ii) temperature, and (iii) pressure.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





