From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of
Question:
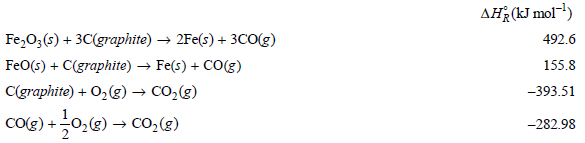
Transcribed Image Text:
AĦ¿(kJ mol) Fe,0;(s) + 3C(graphite) → 2Fe(s) + 3cO(g) FeO(s) + C(graphite) → Fe(s) + CO(g) C(graphite) + O2(g) → CO,(g) CO(e) +0,@) → Co,(3) 492.6 155.8 -393.51 -282.98
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
Fes COg FeOs Cgraphite COg 1 0 8 Cgraphite Og COg ...View the full answer

Answered By

Gauri Hendre
I worked as EI educator for Eduphy India YT channel. I gave online tutorials to the students who were living in the villages and wanted to study much more and were preparing for NEET, TET. I gave tutions for topics in Biotechnology. I am currently working as a tutor on course hero for the biochemistry, microbiology, biology, cell biology, genetics subjects. I worked as a project intern in BAIF where did analysis on diseases mainly genetic disorders in the bovine. I worked as a trainee in serum institute of India and Vasantdada sugar institute. I am working as a writer on Quora partner program from 2019. I writing on the topics on social health issues including current COVID-19 pandemic, different concepts in science discipline. I learned foreign languages such as german and french upto A1 level. I attended different conferences in the science discipline and did trainings in cognitive skills and personality development skills from Lila Poonawalla foundation. I have been the member of Lila poonawalla foundation since 2017. Even I acquired the skills like Excel spreadsheet, MS Office, MS Powerpoint and Data entry.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation for diamond, given that C(graphite) + O2(g) CO2(g) Afr =-393.5 kJ/mol C(diamond) + O2(g) CO2(g) AF1 395.4 kJ/mol
-
From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data Tables), calculate the standard enthalpy of formation of H 2 S(g) and of FeS 2 (s): AR(kJ mol) Fe(s) + 2H2S(g) ...
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
Suppose that today, you paid $1,000 for the bond described in Problem 8 of Chapter 8, The net present value functions: NPV and XNPV. What would be the bonds IRR? A bonds IRR is often called the yield...
-
Formulating an Argument about Earlier American Literature "What question at issue concerning earlier American literature has your research enabled you to identify and answer?" Looking ahead to the...
-
Using comparable transaction analysis, Logans estimate of the fair acquisition value per share for Durtech is closest to: A. \($35.52\). B. \($42.59\). C. \($44.00\). Josh Logan is a buy-side equity...
-
Reproduce Figure 2.4 using geom_histogram(aes(y = ..density..)) as shown below for Example 2.5. ggplot_build() extracts the computed values for the histogram. Use the extracted vales to confirm that...
-
Does competition help or hurt the valuation of a business? Explain.
-
Required information [The following information applies to the questions displayed below] Wemerwoods Company uses a periodic inventory system. It entered into the following purchases and sales...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
Compare the heat evolved at constant pressure per mole of oxygen in the combustion of sucrose (C 12 H 22 O 11 ) and palmitic acid (C 16 H 32 O 2 ) with the combustion of a typical protein, for which...
-
A camper stranded in snowy weather loses heat by wind convection. The camper is packing emergency rations consisting of 58% sucrose, 31% fat, and 11% protein by weight. Using the data provided in...
-
Many countries are lowering taxes on corporations in an effort to be more attractive for investment. In the next table, we list the marginal effective corporate tax rates among Organization for...
-
The inverse of \(A \Rightarrow B\) is the implication \(eg A \Rightarrow eg B\). Explain why the inverse is equivalent to the converse.
-
The equity section of the statement of financial position of Lorenzo pleas at 30 September 2010 is as follows (amounts in thousands of euro): Additional information: Lorenzo plc issued 16,000 shares...
-
The Companies Act 1900 has been referred to as a prominent milestone in the history of company auditing. List reasons which help to explain why this Act has been given this title.
-
A vector field \(\mathbf{F}\) is incompressible if \(\operatorname{div}(\mathbf{F})=0\) and is irrotational if \(\operatorname{curl}(\mathbf{F})=\mathbf{0}\). Prove that the cross product of two...
-
Outline the accounting treatments that are permitted by IAS 19 for actuarial gains and losses in connection with defined benefit pension plans.
-
Sirius B has a mass and radius of 2.1 x 10 30 kg and 5.5 x 10 6 m, respectively. Assuming Sirius B is perfectly spherical in shape, compute the ratio of the t f t / t d . Using this result, confirm...
-
B.) What is the approximate concentration of free Zn 2+ ion at equilibrium when 1.0010 -2 mol zinc nitrate is added to 1.00 L of a solution that is 1.080 M in OH - . For [Zn(OH) 4 ] 2- , K f = 4.610...
-
For how long on average would an atom remain on a surface at 400 K if its desertion activation energy were? (a) 20 kO mol-1, (b) 200 k] mol-I? Take TO = 0.12 ps. For how long on average would the...
-
A solid in contact with a gas at 8.86 kPa and 25C adsorbs 4.67 mg of the gas and obeys the Langmuir isotherm. The enthalpy change when 1.00 mmol of the adsorbed gas is desorbed is +12.2 J. What is...
-
Suppose it is known that ozone adsorbs on a particular surface in accord with a Langmuir isotherm. How could you use the pressure dependence of the fractional coverage to distinguish between...
-
Write down everything you have eaten over a 48 hour time period. Follow the chart below. Date: Times: Breakfast Lunch Dinner Snacks Date: Times: Breakfast Lunch Dinner Snacks
-
Drawing on the knowledge gained in your EVA course, employ relative valuation methods to value any ONE of the given stocks using the data provided in the Assignment Data file. Assume 5% as the...
-
With a few strategies on how the health care facility's leadership team can determine if their changes were successful?

Study smarter with the SolutionInn App


