Predict which of the bent molecules, BH 2 or NH 2 , should have the larger bond
Question:
Figure 24.11
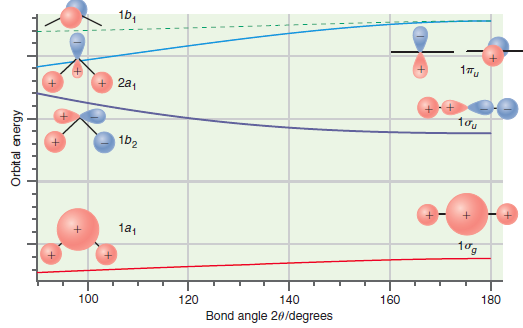
Transcribed Image Text:
16, 1Tu + 2a, 1b2 1a1 180 160 140 100 120 Bond angle 20/degrees Orbital energy
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (15 reviews)
Both molecules are equivalent through the 2a 1 1 orbital Howev...View the full answer

Answered By

Aijaz Khan
I am highly enthusiastic about tutoring. I share a friendly but professional relationship with my students. After completing my electrical engineering I actually taught a course to undergraduates for GATE exam as T.A and it was a brilliant experience and I was one among very few to finish my course on time. I have also helped professors to prepare lessons for my junior fellows while doing my undergrad. Every time I have taught so far, the response has been very heart warming. I hope to continue this and keep on improving it till I am here. Apart from this I have conducted many one on one tutoring lessons.
I believe in focussing on basic and core concepts inorder to keep students' interest alive. My only aim while tutoring is to make student understand concept in such a way that he/she can explain the learnt topic to anyone. Apart from this problem solving is my main focus while tutoring.
I hope to work with SolutionInn for long time. Hope my students will feel the difference.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict whether the ground state or the first excited state of CH 2 should have the larger bond angle on the basis of the Walsh correlation diagram shown in Figure 24.11. Explain your answer. 1b, 17u...
-
Suppose we have some optically pure (R)-2-butyl acetate that has been "labeled" with the heavy 18O isotope at one oxygen atom as shown. (a) Draw a mechanism for the hydrolysis of this compound under...
-
Predict which of the following liquids has greater surface tension: ethanol (C2H5OH) or dimethyl ether (CH3OCH3)?
-
How well employees modify their thoughts and behavior to align with and support a new or changing environment is known as Multiple Choice proactive task performance. proficient task performance....
-
Find the exact values of the remaining trigonometric functions of satisfying the given conditions. 1. tan = 15/8, sin > 0 2. cos = 8/17, tan < 0
-
If the outside air pressure changes from \(1.0 \mathrm{~atm}\) to \(1.1 \mathrm{~atm}\), by how much does mercury in a barometer rise? The mass density of mercury is \(13,534 \mathrm{~kg} /...
-
Describe the importance of a multidisciplinary approach to patient care.
-
Top management of Drexel-Hall is considering closing Store 3. The three stores are close enough together that management estimates closing Store 3 would cause sales at Store 1 to increase by $60,000,...
-
Give the big O notation (as a function of n) of the running times of the following code fragment: int sum = 0; for (int j = n;j> 0; j = j / 3) sum++;
-
2. Calculate the production cost per unit for each of Harbours products under a traditional costing system. 3. Calculate Harbours gross margin per unit for each product under the traditional costing...
-
Show that the water hybrid bonding orbitals given by a = 0.55 2Pz + 0.71 2 px -0.45 2 ps b = 0.55 2pz - 0.71 2px - 0.45 2s are orthogonal.
-
Derive two additional mutually orthogonal hybrid orbitals for the lone pairs on oxygen in H 2 O, each of which is orthogonal to Ï a and Ï b , by following these steps: a. Starting with the...
-
Jays Appliances sells micro-fridges according to the monthly demand distribution shown in the table at the top of the next page. Simulate 6 years of demand and compare theoretical and simulated...
-
Which of the following items does not normally enter into the computation of distributable net income? a. Charitable deduction b. Capital gains c. Trustees commissions d. Rental income
-
Illustrate the Distinctive characteristics of services (customer participation, simultaneity, perishability, intangibility, heterogeneity) for the United States Postal Service Operation.
-
How can communication barriers in health care teams, such as hierarchy, status, and other factors, be addressed in hospitals and other healthcare organizations? How can communication experts persuade...
-
Which of the following items affects the charitable deduction of an estate or complex trust? a. Rental income b. Dividends c. Interest d. Tax-exempt interest
-
Depression can be considered as a set of symptoms or as an interpersonal/ relational concern. What issues arise from considering depression from each perspective? Are there similarities and...
-
In problem, determine whether the given function is linear, exponential, or neither. For those that are linear functions, find a linear function that models the data; for those that are exponential,...
-
To balance the chemical equation SiH3 + O2 SiO2 + HO, you could introduce coefficients a, b, c, d and write aSiH3 + bO2 cSiO + dHO then write linear equations for each element. The equation for Si...
-
Derive an expression for the time dependence of the degree of polymerization for a stepwise polymerization in which the reaction is acid catalysed by the -COOH acid functional group. The rate law is...
-
Calculate the average polymer length in a polymer produced by a chain mechanism in which termination occurs by a disproportionation reaction of the form M + M M + :M.
-
Calculate the ratio of the mean cube molar mass to the mean square molar mass in terms of (a) The fraction p, (b) The chain length.
-
Use matrices (row-echelon form) to solve the following system of linear equa- tions. If the system has no solution, say that it is inconsistent. 3x + 2y = 7 x+y=3
-
For the following function : f(x) = 1/3 x^3 + 4x^2 + 16x a. Determine the critical points. b. Calculate the second derivative c. Determine if the function is concave up or down or not concave d....
-
2. An activity director for a cruise ship has surveyed 240 passengers. Of the 240 passengers; 135 like swimming, 150 like dancing, 65 like games, 80 like swimming and dancing, 40 like swimming and...

Study smarter with the SolutionInn App


