Purification of proteins for use as biopharmaceuticals is often accomplished by ion exchange chromatography, in which a
Question:
Purification of proteins for use as biopharmaceuticals is often accomplished by ion exchange chromatography, in which a process fluid passes through a column packed with small resin beads whose ionic surface charge causes them to adsorb some stream components more strongly than others. An ion-exchange run takes place in two steps: (1) the load step, in which the process stream flows through the column and the target protein (the product) and some undesired impurities are adsorbed onto the resin; and (2) the elution step, during which another fluid passes through the column and desorbs the impurities and the protein from the resin. The elution fluid consists of an aqueous solution of a solute known as Tris diluted with an NaCl solution, with the NaCl-to-Tris ratio starting at 0 and steadily increasing with time. The impurities desorb into the fluid when the NaCl concentration is low, and the effluent is collected in a waste vessel. As the NaCl concentration increases, the target protein desorbs. When analysis of the effluent reveals the presence of the target protein, the flow is switched to the product collection vessel, and the effluent is collected until no more product is detected in the effluent. The collected product is then subjected to additional process steps to further isolate the protein, and the column is cleaned for reuse.
Consider an elution step in which solutions of 1MNaCl (solution A) and 50mMTris (solution B) are mixed and fed to a loaded ion-exchange column. The system is programmed to keep the total volumetric flowrate (V̇A + V̇B) into the column constant at 120 L/h while linearly increasing the volume fraction of solution A in the feed from0%to 20%over a period of 33.6 minutes, at which point the elution is declared to be complete. The flowchart is shown below:
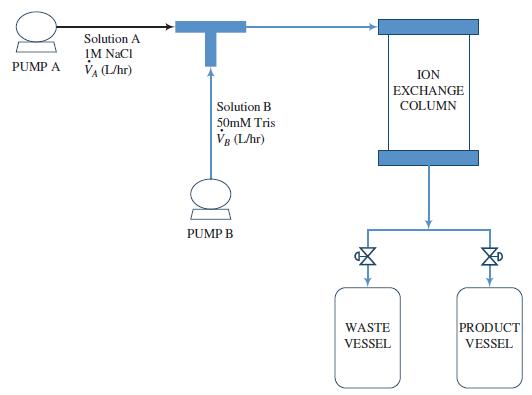
(a) Calculate Vt(L), the total amount of solution fed to the column.
(b) Derive an equation for the volumetric flow rate of solution A, V̇A(t), assuming that the densities of both fluids are the same. Use the calculated value to determine calculate VA(L), the total volume of that solution fed to the column, and mA(g NaCl), the total mass of NaCl fed. Then determine V̇B(t), and VB(L) and nB(mol Tris), the total volume of solution B and total moles of Tris fed, respectively. Once you’ve done the calculations for solution A, those for B should be trivial.
(c) Suppose in one run product is detected in the effluent at the same time impurities are detected— that is, product protein starts desorbing earlier than in previous runs. List up to five possible causes of the problem.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





