Pyramidal inversion in the cyclic amine aziridine is significantly more difficult than inversion in an acyclic amine;
Question:
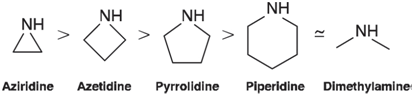
Optimize the geometries of aziridine, azetidine, pyrrolidine, and piperidine using the HF/6-31G* model. Starting from these optimized structures, provide guesses at the respective inversion transition states by replacing the tetrahedral nitrogen center with a trigonal center. Obtain transition states using the same Hartree€“Fock model and calculate inversion barriers. Calculate vibrational frequencies to verify that you have actually located the appropriate inversion transition states. Do the calculated inversion barriers follow the order suggested in the preceding figure? If not, which molecule(s) appear to be anomalous? Rationalize your observations by considering other changes in geometry from the amine to the transition state.
Step by Step Answer:






