Seawater containing 3.5 wt% dissolved salts is to be desalinated in an adiabatic six-effect evaporator. Backward feed
Question:
Seawater containing 3.5 wt% dissolved salts is to be desalinated in an adiabatic six-effect evaporator. Backward feed is to be used: the seawater is fed to the last evaporator, and successively concentrated brine solutions flow countercurrent to the direction of flow of steam from one effect to the next. Saturated steam at P = 2 bar is fed to the tube bundle in the first effect. The operating pressures in bars of the six effects are, respectively, 0.9, 0.7, 0.5, 0.3, 0.2, and 0.1. The brine leaving the first effect contains 30 wt% salt. The flowchart shows Effects 1, 5, and 6.
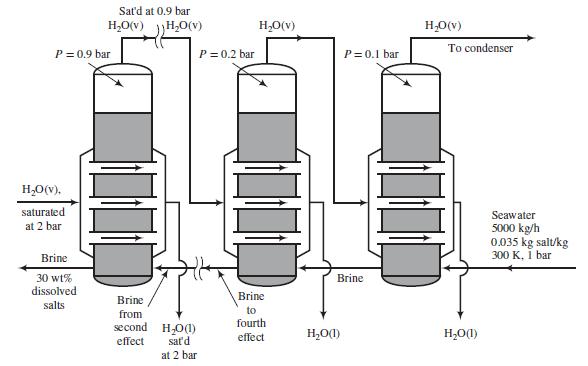
Following is a labeled diagram of the ith effect:
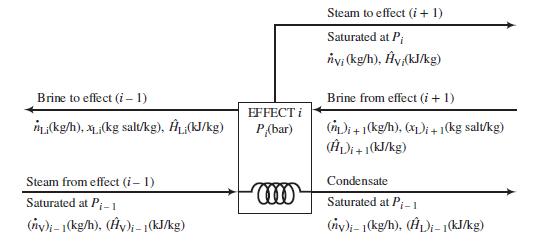
In terms of the variables defined in this diagram,
nL7 = 5000 kg/h
x L7 = 0:035 kg salt/kg
x L1 = 0:30 kg salt/kg
n̂V0 = feed rate of steam to the first effect
(a) Use a salt balance to calculate n̂L1. Then use this result to determine how much fresh water is produced in the process.
(b) Prepare a table as follows:
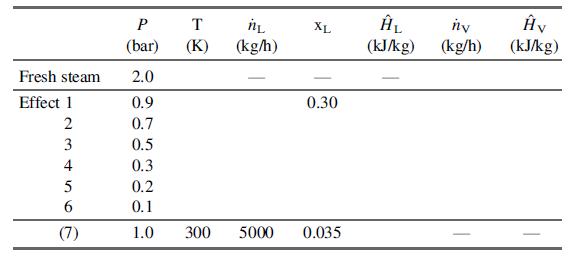
Fill in all known variable values (do not calculate any yet), including values obtained from the steam tables, assuming that the physical properties of the brine solution are those of pure water.
(c) Show that the following equations can be derived from balances:
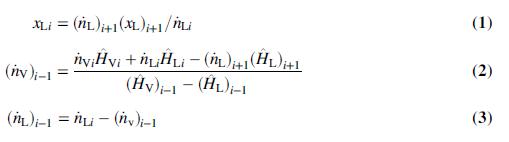
(d) Solve the equations of Part (c) for all six effects using Excel’s Solver or another equation solving program. Fill in the table of Part (b).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




