Several reactions and their standard reaction enthalpies at 298.15 K are given here: The standard enthalpies of
Question:
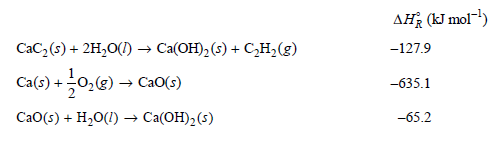
The standard enthalpies of combustion of graphite and C2H2(g) are €“393.51 and €“1299.58 kJ mol€“1, respectively. Calculate the standard enthalpy of formation of CaC2(s) at 25°C.
Transcribed Image Text:
AH (kJ mol-) -127.9 CaC,(s) + 2H,O(1) → Ca(OH),(s) + CH,(g) Ca(s) + 0,(g) → Cao(;) -635.1 CaO(s) + H20(1) → Ca(OH)2(s) -65.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 86% (15 reviews)
CaOH s CHg CaC s 2HO1 CaOs HO1 ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The half-reactions involved in the lactate dehydrogenase (LDH) reaction and their standard reduction potentials are Calculate (G at pH 7.0 for the LDH-catalyzed reduction of pyruvate under the...
-
Given the following reactions and their enthalpies: a. Devise a way to calculate H for the reaction H2O(g) 2H(g) + O(g) b. From this, estimate the H - O bond energy. (kJ/mol) +436 +495 H2(g)- 2H(g)...
-
The standard free energies of formation and the standard enthalpies of formation at 298 K for difluoroacetylene (C2F2) and hexafluorobenzene (C6F6) are For the following reaction: C6F6(g) 3C2F2(g) a....
-
Find the direction cosines and angles of, and demonstrate that the sum of the squares of the direction cosines is 1. u = 5i + 3j - k
-
What zoning ordinances, if any, regulate the type of home-based business you want to start?
-
Rank in order, from greatest to least, the kinetic energies of the sliding pucks. 1 kg 2 m/s 1 kg 3 m/s A. B. -2 m/s 1 kg 2 kg 2 m/s C. D.
-
Consider the life expectancy data given in Problem 3.16 and Table B.16. Problem 3.16 Rossman [1994] presents an interesting study of average life expectancy of 40 countries. Table B. 16 gives the...
-
Kara Fashions uses straight-line depreciation for financial statement reporting and MACRS for income tax reporting. Three years after its purchase, one of Kara's buildings has a carrying value of...
-
The number of customers arriving at Soda Mart follows a Poisson distribution with a mean of 22 customers per hour. What is the probability 40 customers will arrive in two (2) hours?
-
A counter flowing heat exchanger is used to cool air at 540 K, 400 kPa to 360 K by using a 0.05 kg/s supply of water at 20C, 200 kPa. The air flow is 0.5 kg/s in a 10-cm diameter pipe. Find...
-
Draw structures for any five constitutional isomers with molecular formula C 2 H 6 O 3 ?
-
For each type of bond below, determine the direction of the expected dipole moment. a) C O b) C Mg c) C N d) C Li e) C Cl f) C H g) O H h) N H
-
In June 1993, Sparkomatic Corp. agreed to negotiate a sale of its Kenco Engineering division to Williams Controls, Inc. At the end of July, Sparkomatic asked its accountants, Parente, Randolph,...
-
As we have seen many times throughout this text, the law is a balancing act. We have just examined how the courts have been willing (eager?) to use strict liability to hold manufacturers and...
-
Canadas real GDP was $1,830 billion in 2017 and $1,866 billion in 2018. Canadas population was 36.7 million in 2017 and 37.1 million in 2018. Calculate: a. The growth rate of real GDP. b. The growth...
-
The following figure shows the market for lows killed labor. The value of marginal product of high-skilled workers is $16 an hour greater than that of lows killed workers at each quantity of labor....
-
What are the rights and duties of sellers and buyers in a sales contract?
-
Explain how the pursuit of profit can sometimes lead to bank failures. U.S. bank profits rose during the first quarter of 2021, up $58.3 billion from a year ago. Loan balances decreased and deposits...
-
Find two negative and three positive angles, expressed in radians, for which the point on the unit circle that corresponds to each angle is (-2/2, 2/2).
-
Whats the difference between an ordinary annuity and an annuity due? What type of annuity is shown below? How would you change the time line to show the other type of annuity?
-
The emission spectrum of a porphyry in dissolved in O,-saturated water shows a strong band at 650 nm and a weak band at 1270 nm. In separate experiments, it was observed that the electronic...
-
Use the Chapman model to explore the behaviour of a model atmosphere consisting of pure 0, at 10 Torr and 298 K that is exposed to measurable frequencies and intensities of UV radiation. (a) Look up...
-
Because of its importance in atmospheric chemistry, the thermal decomposition of nitric oxide, 2 NO (g) -7 N,(g) + O,(g), has been amongst the most thoroughly studied of gas-phase reactions. The...
-
Explore the concept of principal quantum numbers in quantum mechanics and atomic structure. How do principal quantum numbers, denoted by the symbol "n," define the energy levels and electron...
-
1. How many electrons make up a charge of 1.0 MC? 2. What is the total charge on 1.0 kg of electrons?
-
What frequency of sound would have a wavelength the same size as a 0.81 m -wide window? (The speed of sound is 344m/s at 20 o C .)

Study smarter with the SolutionInn App


