Singlet and triplet carbenes exhibit different properties and show markedly different chemistry. For example, a singlet carbene
Question:
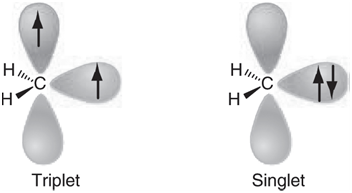
It should be possible to take advantage of what we know about stabilizing radical centers versus stabilizing empty orbitals and use that knowledge to design carbenes that will either be singlets or triplets. Additionally, it should be possible to say with confidence that a specific carbene of interest will either be a singlet or a triplet and, thus, to anticipate its chemistry. The first step is to pick a model and then to establish the error in the calculated singlet€“triplet energy separation in methylene, where the triplet is known experimentally to be approximately 42 kJ/mol lower in energy than the singlet. This can then be applied as a correction for calculated singlet€“triplet separations in other systems.
a. Optimize the structures of both the singlet and triplet states of methylene using both Hartree€“Fock and B3LYP density functional models with the 6-31G* basis set. Which state (singlet or triplet) is found to be of lower energy according to the HF/6-31G* calculations? Is the singlet or the triplet unduly favored at this level of calculation? Rationalize your result. (Triplet methylene contains one fewer electron pair than singlet methylene.) What energy correction needs to be applied to calculated singlet€“triplet energy separations? Which state (singlet or triplet) is found to be of lower energy according to the B3LYP/6-31G* calculations? What energy correction needs to be applied to calculated energy separations?
b. Proceed with either the HF/6-31G* or B3LYP/6-31G* model, depending on which leads to better agreement for the singlet€“triplet energy separation in methylene. Optimize singlet and triplet states for cyanomethylene, methoxymethylene, and cyclopentadienylidene Apply the correction obtained in the previous step to estimate the singlet€“triplet energy separation in each. For each of the three carbenes, assign the ground state as singlet or triplet. Relative to hydrogen (in methylene), has the cyano substituent in cyanomethylene and the methoxy substituent in methoxymethylene led to favoring of the singlet or the triplet? Rationalize your result by first characterizing cyano and methoxy substituents as Ï€ donors or Ï€ acceptors, and then speculating about how a donor or acceptor would stabilize or destabilize singlet and triplet methylene. Has incorporation into a cyclopentadienyl ring led to increased preference for a singlet or triplet ground state (relative to the preference in methylene)? Rationalize your result. (Count the number of Ï€ electrons associated with the rings in both singlet and triplet states.
Step by Step Answer:






