The composition of a coal is determined by a proximate analysis. The coal is first finely ground
Question:
The composition of a coal is determined by a proximate analysis. The coal is first finely ground and air-dried. Samples of the dried coal are then subjected to several operations, with the sample weights being recorded before and after each operation. Moisture content is determined as the weight loss when a sample is held at 105°C in an oxygen-free atmosphere for roughly 2 h, added to the weight loss in the initial drying step. Volatile matter (primarily organic tars) is determined by holding a sample at 925°C in an oxygen-free atmosphere for 7 min and subtracting the moisture loss from the total weight loss. Ash (or mineral matter—oxides and sulfates of silicon, aluminum, iron, calcium, sulfur, and trace minerals) is the residue that remains after a sample has been heated to 800°C in an oxygen-containing atmosphere until all the organic matter has been burned away. Fixed carbon is what is present in coal besides moisture, volatile matter, and ash.
(a) Use the following proximate analysis data to determine the percentages by mass of moisture, fixed carbon, volatile matter, and ash in a coal:
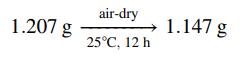
The remaining tests are performed on air-dried samples.
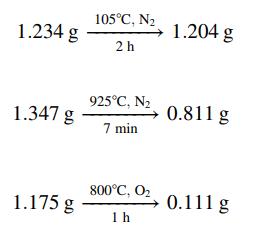
(b) If the mass ratio of C to H in the volatile matter is 6:1, calculate the gram-moles of air theoretically required to burn 1 metric ton of this coal.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard




