The equilibrium between hydrogen cyanide (HCN) and its isomer hydrogen isocyanide (HNC) is important in interstellar chemistry:
Question:
HCN (g) ‡„ HNC (g)
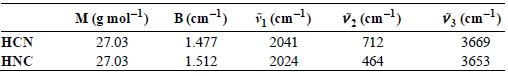
A long-standing €œpuzzle€ regarding this reaction is that in space (T = 2.75 K) surprisingly large amounts of HNC are observed. For example, HNC/HCN ratios approaching 20% have been observed in comets (Advances in Space Research, 31 [2003]: 2557). Using the spectroscopic information provided below and knowledge that the potential-energy surface minimum of HNC lies roughly 5200 cmˆ’1 higher in energy relative to HCN, calculate the theoretical value for KP for this reaction occurring in interstellar space.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





