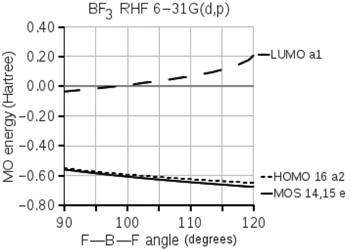
The following diagram shows the energies of valence molecular orbitals of boron trifluoride. The energies of three
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





