The gas-phase decomposition of ethyl bromide is a first-order reaction, occurring with a rate constant that demonstrates
Question:
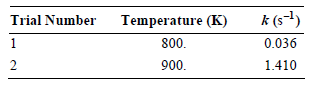
a. Determine the Arrhenius parameters for this reaction.
b. Using these parameters, determine ΔH€¡ and ΔS€¡ as described by the Eyring equation.
Transcribed Image Text:
k (s-l) Trial Number Temperature (K) 0.036 800. 900. 1.410
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a The activation energy is determined by taking the ratio of the rate constan...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The unimolecular decomposition of urea in aqueous solution is measured at two different temperatures and the following data are observed a. Determine the Arrhenius parameters for this reaction. b....
-
Hydrogen abstraction from hydrocarbons by atomic chlorine is a mechanism for Cl · loss in the stratosphere. Consider the reaction of Cl· with ethane: C 2 H 6 (g) + Cl· (g) C 2 H...
-
Chlorine monoxide (ClO i ) demonstrates three bimolecular self-reactions: The following table provides the Arrhenius parameters for this reaction: a. For which reaction is ÎH ¡ greatest...
-
answer the following questions about the countries below using CIA factbook Economic Systems Continuum Activity: Country Information Cards Answer the following questions about the countries below...
-
Find each function value. 1. f (x) = |x + 2| - 15, (a) f (-8) (b) f (14) (c) f (x - 6) 2. f (x) = (x + 9) / (x2 - 81) (a) f (7) (b) f (-5) (c) f (x - 9)
-
The following information has been compiled from the accounting records of Benji Ltd for the year ended 30 June 2023 in relation to its financing activities. Required Determine the amount of net cash...
-
Consider the patient satisfaction data in Table B.17. For the purposes of this exercise, ignore the regressor "Medical-Surgical." Perform a thorough analysis of these data. Please discuss any...
-
Implement the following LP model in a spreadsheet. Use Solver to solve the problem and create a Sensitivity Report. Use this information to answer the following questions: MIN: 5X1 + 3X2 + 4X3...
-
1. You need to modify an existing VHD file. Q: How do you proceed? 2. A web-based site is needed by all users on the company's network in order to perform research on the company's competitors. After...
-
"Juniors Network" Cartoon Channel is the favorite channel of all the kids in the city. As it is vacation time, the channel had introduced several new segments to engage the kids in a more creative...
-
Catalase is an enzyme that promotes the conversion of hydrogen peroxide (H 2 O 2 ) into water and oxygen. The diffusion constant and radius for catalase are 6.0 10 7 cm 2 s 1 and 51.2 ,...
-
Consider the unimolecular isomerization of methylcyanide, a reaction that will be discussed in detail in Chapter 36: CH 3 NC(g) CH 3 CN(g) The Arrhenius parameters for this reaction are A = 2.5 10...
-
Human safeguards are often taken for granted, but they are important. Read about the famous embezzlement in Oregon by Elma Magkamit. What safeguards would have prevented her from stealing money from...
-
Explain how each of the following events affects the monetary base, the money multiplier, and the money supply. a. The Federal Reserve buys bonds in an openmarket operation. b. The Fed increases the...
-
The imposition of a tax _________. a) raises both supply and demand b) lowers neither supply nor demand c) lowers only supply d) lowers only demand
-
Find data on GDP and its components, and compute the percentage of GDP for the following components for 1950, 1980, and the most recent year available. a. Personal consumption expenditures b. Gross...
-
When quantity demanded is greater than quantity supplied, _______. a) market price will rise b) market price will fall c) market price will stay the same
-
As the price of a service rises, _______. a) the consumer surplus decreases b) the consumer surplus increases c) the consumer surplus may increase or decrease
-
On January 1, 2020, Jade Company issued $2,000,000 face value, 7%, 10-year bonds at $2,147,202. This price resulted in a 6% effective-interest rate on the bonds. Jade uses the effective-interest...
-
A certain Christmas tree ornament is a silver sphere having a diameter of 8.50 cm. Determine an object location for which the size of the reflected image is three-fourths the size of the object. Use...
-
As shown in Example Problem 3.5, (U m /V) T a/V 2 m for a van der Waals gas. In this problem, you will compare the change in energy with temperature and volume for N 2 , treating it as a van der...
-
Calculate H o f for NO(g), at 975 K, assuming that the heat capacities of reactants and products are constant over the temperature interval at their values at 298.15 K.
-
Prove that C V = - (U/V) T (V/T) U.
-
Victor Korchnoi bought a bond one month before a semi-annual coupon was due. The face value was $10,000 and the coupon rate 8.5%. At the time of purchase there were 34 coupons left and the YTM was 6%...
-
4. Consider a 30-year U.S. Treasury bond paying 4.5 percent coupon, and selling for $1010. What is the yield to maturity? Make sure to show your work. 5. A 30-year U.S. corporate bond with a 6...
-
A project's base case or most likely NPV is $44,000, and assume its probability of occurrence is 50%. Assume the best-case scenario NPV is 65% higher than the base case and assume the worst scenario...

Study smarter with the SolutionInn App


