The observed lines in the emission spectrum of atomic hydrogen are given by In the notation favored
Question:
The observed lines in the emission spectrum of atomic hydrogen are given by
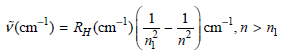
In the notation favored by spectroscopists, ν̅ = 1/λ = E/hc and RH =109,677 cm−1. The Lyman, Balmer, and Paschen series refers to n1 = 1, 2, and 3, respectively, for emission from atomic hydrogen. What is the highest value of ν???? and E in each of these series?
Transcribed Image Text:
-1 cm,n > m v(cm) = R#(cm) т
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The highest value for corresponds to 1n 0 Therefore V ...View the full answer

Answered By

Geoffrey Isaboke
I am an industrious tutor with a 5-yr experience in professional academic writing. I have passion for History and Music and I have good knowledge in Economics
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The visible emission lines observed by Balmer all involved nf = 2. (a) Explain why only the lines with nf = 2were observed in the visible region of the electromagnetic spectrum. (b) Calculate the...
-
The Humphreys series is a group of lines in the spectrum of atomic hydrogen. It begins at 12 368 nm and has been traced to 3281.4 nm. What are the transitions involved? What are the wavelengths of...
-
In the line spectrum of atomic hydrogen, there is also a group of lines known as the Fund series. These lines are produced when electrons, excited to high energy levels, make transitions to the n = 5...
-
If the price increased by 40%, what will happen to EOQ? (Assumed the original demand level and ordering cost remain constant). What is the elasticity of EOQ with respect to price? (That is, the...
-
Four surgical procedures currently are used to install pacemakers. If the patient does not need to return for follow-up surgery, the operation is called a "clear" operation. A heart center wants to...
-
A typical meteor that hits the earths upper atmosphere has a mass of only 2.5 g, about the same as a penny, but it is moving at an impressive 40 km/s. As the meteor slows, the resulting thermal...
-
A project has a first cost of $\$ 16,999$ with a life of 10 years and a salvage value of $\$ 2,500$. If the MARR is $12 \%$ and provides benefits of about $\$ 2,750$ annually, what is the...
-
Giant acquired all of Small's common stock on January 1, 2011. Over the next few years, Giant applied the equity method to the recording of this investment. At the date of the original acquisition,...
-
A bond is currently trading at a YTM of 14%. We also know that its market price is $892, and that its coupon rate is 5%. What is the bond's expected Capital Gains Yield?
-
General Motors Accounting Failures the below needs to answer Background of the topic The main reasons for the Recent failings The impact and consequences of the failures The role of Accountants The...
-
Develop life tables for subsets of the data based on age, gender, number of cigarettes per day, and CO level (one variable at a time). Given these data, do you feel age, gender, number of cigarettes...
-
Refer to the following table. Find out the rate of growth of expenditure on durable goods. What is the estimated semielasticity? Interpret your results. Would it make sense to run a double log...
-
During 2010. Kirkpatrick Corporation purchased a new electric generator for $2,750,000. The generator is expected to have a five-year useful life and will be disposed of in 2015 without any...
-
Melissa owns a house which she lets to tenants. The house was let througho ut tax year 2017-18 at a rent of 600 per month . Her allowable expenditure in 2017-18 was 900 and she had property losses...
-
Ivy begins trading as a farmer on 1 January 201 5, making up annual accounts to 31 December. Her adjusted trading profits/(losses) in the opening years are as follows: (a) (b) year to 31 December...
-
Brian and Danny are civil partners. They were both born in 1974 and they both claim the basic personal allowance for 2017 -18. Brian's total income for 201 7-18 is 25,000 and Danny's total income for...
-
Stephanie (who is not a Scottish taxpayer) has the following income in 2017-18: Income from self-employment 28,880 Rents received 15,730 Bank interest 200 Dividends 250 Compute Stephanie's income tax...
-
Ernest (who is not a Scottish taxpayer) has a retirement pension in 2017-18 of 51,890 and bank interest of 620.His personal allowance for the year is 11,5 00. ComputeErnest's income tax liability...
-
Lines l 1 and l 2 are parallel. Determine the measures of 1 through 12. 45 59 6/70 9/10 11\12
-
Conduct a VRIO analysis by ranking Husson University (in Maine) business school in terms of the following six dimensions relative to the top three rival schools. If you were the dean with a limited...
-
Explore whether a magnetic field can influence the heat capacity of a paramagnetic molecule by calculating the electronic contribution to the heat capacity of an NO 2 molecule in a magnetic field....
-
Consider Stirlings approximation for ln N! in the derivation of the Boltzmann distribution. What difference would it make if (a) A cruder approximation, N! = N N , (b) The better approximation in...
-
A certain molecule can exist in either a non-degenerate singlet state or a triplet state (with degeneracy 3). The energy of the triplet exceeds that of the singlet by . Assuming that the molecules...
-
Two point charges, -2.4C and 5.616 C, are placed at x = 0 cm and x = 9.7 cm, respectively. Consider the x- axis directed to the right. 1.0p 8a At what point along the x axis is the electric field...
-
As you know, the value of. It is defined as the ratio of the circumference of a circle C divided by its diameter 2r. That is x-C/2. a) Let's assume that you measured a circumference of a circle to be...
-
Figure 3 presents a network where N users are sharing a link of 1 Mbps bandwidth. Users are generating data at a rate of 100 kbps when busy, but are busy generating data only with probability p=0.1....

Study smarter with the SolutionInn App


