The reaction of nitric oxide (NO(g)) with molecular hydrogen (H2(g)) results in the production of molecular nitrogen
Question:
2NO(g) + 2H2(g) †’ N2O(g) + 2H2O(g)
The experimentally determined rate law expression for this reaction is first order in H2(g) and second order in NO(g).
a. Is the reaction as written consistent with the experimental order dependence for this reaction?
b. One potential mechanism for this reaction is as follows:
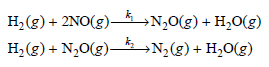
Is this mechanism consistent with the experimental rate law?
c. An alternative mechanism for the reaction is:
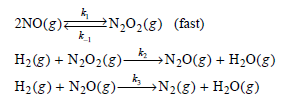
Show that this mechanism is consistent with the experimental rate law.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





