The shells of marine organisms contain calcium carbonate CaCO 3 , largely in a crystalline form known
Question:
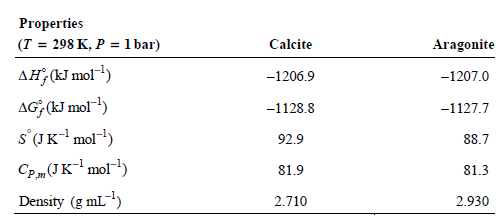
a. Based on the thermodynamic data given, would you expect an isolated sample of calcite at T = 298 K and P = 1 bar to convert to aragonite, given sufficient time? Explain.
b. Suppose the pressure applied to an isolated sample of calcite is increased. Can the pressure be increased to the point that isolated calcite will be converted to aragonite? Explain.
c. What pressure must be achieved to induce the conversion of calcite to aragonite at T = 298 K? Assume both calcite and aragonite are incompressible at T = 298 K.
d. Can calcite be converted to aragonite at P = 1.00 bar if the temperature is increased? Explain.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





