The three vibrational frequencies in H 2 O (1595, 3657, and 3756 cm 1 ) are all
Question:
This follows from the fact that vibrational frequency is given by the square root of a (mass-independent) quantity, which relates to the curvature of the energy surface at the minima, divided by a quantity that depends on the masses of the atoms involved in the motion.
As discussed in Section 26.8.4, vibrational frequencies enter into both terms required to relate the energy obtained from a quantum chemical calculation (stationary nuclei at 0 K) to the enthalpy obtained experimentally (vibrating nuclei at finite temperature), as well as the entropy required to relate enthalpies to free energies. For the present purpose, focus is entirely on the so-called zero point energy term; that is, the energy required to account for the latent vibrational energy of a molecule at 0 K.
The zero point energy is given simply as the sum over individual vibrational energies (frequencies). Thus, the zero point energy for a molecule in which isotopic substitution has resulted in an increase in mass will be reduced from that in the un-substituted molecule:

direct consequence of this is that enthalpies of bond dissociation for iso-topically substituted molecules (light to heavy) are larger than those for un-substituted molecules.
a. Perform B3LYP/6-31G* calculations on HCl and on its dissociation products, chlorine atom and hydrogen atom. Following geometry optimization on HCl, calculate the vibrational frequency for both HCl and DCl and evaluate the zero point energy for each. In terms of a percentage of the total bond dissociation energy, what is the change noted in going from HCl to DCl?
b. (dichloromethyl radical) the zero point energy of the products of hydrogen abstraction (DCCl2 + HCl) is 51 kJ/mol and that of the products of deuterium abstraction (HCCl2 + DCl) is 54 kJ/mol. Thus, hydrogen abstraction is favored thermodynamically. The Boltzmann ratio of hydrogen to deuterium abstraction products is roughly 90:10 at room temperature.
c. Obtain the transition state for hydrogen abstraction from methylene chloride using the B3LYP/6-31G* model. A reasonable guess is shown here:
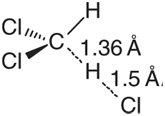
Calculate vibrational frequencies for the two possible structures with one deuterium and evaluate the zero point energies for these two structures. (For the purpose of zero point energy calculation, ignore the imaginary frequency corresponding to the reaction coordinate.) Which pathway is favored on the basis of kinetics? Is it the same as or different from the thermodynamic pathway? What would you expect the (kinetic) product ratio to be at room temperature?
Step by Step Answer:






