The unimolecular decomposition of urea in aqueous solution is measured at two different temperatures and the following
Question:
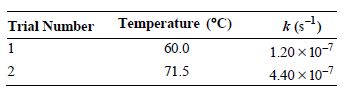
a. Determine the Arrhenius parameters for this reaction.
b. Using these parameters, determine ΔH€¡ and ΔS€¡ as described by the Eyring equation.
Transcribed Image Text:
Temperature (°C) k (s-) Trial Number 60.0 1.20 x 10-7 71.5 4.40 x 10-7 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
a The activation energy is determined by taking the ratio of the rate constants ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Hydrogen abstraction from hydrocarbons by atomic chlorine is a mechanism for Cl · loss in the stratosphere. Consider the reaction of Cl· with ethane: C 2 H 6 (g) + Cl· (g) C 2 H...
-
Suppose the vapor pressure of a substance is measured at two different temperatures. (a) By using the Clausius-Clapeyron equation (Equation 11.1) derive the following relationship between the vapor...
-
The gas-phase decomposition of ethyl bromide is a first-order reaction, occurring with a rate constant that demonstrates the following dependence on temperature: a. Determine the Arrhenius parameters...
-
why is teamwork so important especially in healthcare? explain
-
Find (a) the domain and (b) the zeroes of the function. 1. f (x) = (x -5) / (2x2 - x) 2. f (x) = 10 - (3 - x)
-
What disclosures are required by AASB 101/IAS 1 regarding accounting policies?
-
Consider the fuel consumption data in Table B.18. For the purposes of this exercise, ignore regressor $x_{1}$. Perform a thorough analysis of these data. What conclusions do you draw from this...
-
In early 20X1, SpaceTel Communications, a U.S.-based international telephone communications company, purchased the controlling interest in Sofia Telecom, Ltd. (STL) in Bulgaria. A key productivity...
-
This house is new and how to set up the network in this house? CLOSET BATH 3 CLOS MASTER BATH SITTING 8'6"X7'0" BALCONY 10'4"x5'8" MASTER WARDROBE MASTER SUITE 18'0"x14'0" UTILITY LIFT DEN...
-
From the following T accounts of Linda's Consulting Service of Taber, (a) Record and foot the balances on the appropriate pages in the Study Guide with Working Papers, and (b) Prepare a trial balance...
-
Catalase is an enzyme that promotes the conversion of hydrogen peroxide (H 2 O 2 ) into water and oxygen. The diffusion constant and radius for catalase are 6.0 10 7 cm 2 s 1 and 51.2 ,...
-
Consider the unimolecular isomerization of methylcyanide, a reaction that will be discussed in detail in Chapter 36: CH 3 NC(g) CH 3 CN(g) The Arrhenius parameters for this reaction are A = 2.5 10...
-
When recording the disposal of a long-term operating asset, why is it necessary to debit the accumulated depreciation of the old asset?
-
Which statement is true? a) A price floor is above equilibrium price and causes surpluses. b) A price floor is above equilibrium price and causes shortages. c) A price floor is below equilibrium...
-
We learned that Facebook acquired GIPHY for a sum of $400 million. Facebook has historically maintained its WACC at around 8%. Assume that Facebook acquired another app this year for $500 million and...
-
Supply is most elastic in ______. a) the market period b) the short run c) the long run
-
If the price system is allowed to function without interference and a shortage occurs, quantity demanded will __________ and quantity supplied will _____as the price rises to its equilibrium level....
-
A tax will ______. a) lower price and raise supply b) lower price and lower supply c) raise price and lower supply d) raise price and raise supply
-
Suppose the 2020 adidas financial statements contain the following selected data (in millions). Compute the following values and provide a brief interpretation of each. a. Debt to assets ratio. b....
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Given its mission of providing information to the world, should Google censor searches in China?
-
At 298 K and 1 bar pressure, the density of water is 0.9970 g cm 3 , and C P,m = 75.3 J K -1 mol -1 . The change in volume with temperature is given by V = V i T, where , the coefficient of thermal...
-
5-Methylcyclopentadiene undergoes homolytic bond cleavage of a C!H bond to form a radical that exhibits five resonance structures. Determine which hydrogen is abstracted and draw all five resonance...
-
Calculate S surroundings and S total for the processes described in parts (a) and (b) of Problem P5.16. Which of the processes is a spontaneous process? The state of the surroundings for each part is...
-
The following information pertains to the inventory of Parvin Company: Jan. 1 Apr. 1 Oct. 1 Beginning inventory Purchased Purchased 400 units @ 2,400 units @ 1,100 units $17 $22 $23 During the year,...
-
Gold Nest Company of Guandong, China, makes birdcages for the South China market. The company sells its birdcages through an extensive network of street vendors who receive commissions on their...
-
Sandy Bank, Incorporated, makes one model of wooden canoe. Partial information is given below. Required: 1. Complete the following table. 2. Suppose Sandy Bank sells its canoes for $510 each....

Study smarter with the SolutionInn App


