The vapor pressure of liquid benzene is 20,170 Pa at 298.15 K, and Î H vaporization =30.72
Question:
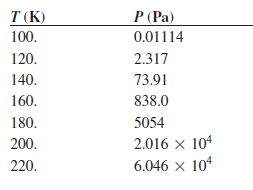
Transcribed Image Text:
T (K) P (Pa) 100. 0.01114 2.317 120. 73.91 140. 838.0 160. 180. 5054 200. 2.016 x 104 6.046 x 104 220.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
At the normal boiling point P 101325 Pa At the standard boiling point P 10 ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
At 298.15 K, G f (HCOOH, g) = -351.0 kJ mol -1 and G f (HCOOH, l) 361.4 kJ mol -1 . Calculate the vapor pressure of water at this temperature.
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
The vapor pressure of ethanol(l) is given by a. Calculate the standard boiling temperature. b. Calculate ÎH vaporization at 298 K and at the standard boiling temperature. 3.6745 x 10 23.58 In...
-
Determine the range of the 2x function y = 3 sec 3
-
Write a short Network Management Paper: In which, you will research and report on network management tools associated with: (1) Policy compliance, (2) Bandwidth management, (3) Asset management....
-
What does it mean when we say that \(t\)-test statistics are marginal?
-
Water is flowing in a pipe of diameter \(20 \mathrm{~cm}\) with a pressure gradient of \(3000 \mathrm{~Pa} / \mathrm{m} . \mu=\) \(0.001 \mathrm{~Pa} \cdot \mathrm{s}\). Find the wall shear stress....
-
An electron's position is given by r = 3.00ti 4.00t 2 j + 2.00k, with t in seconds and r in meters. (a) In unit vector notation, what is the electron's velocity v (t)? At t = 2.00 s, what is v (b)...
-
Please provide your prediction for Apple sales for the year ending in September 2 0 2 4 ? Do you think Apple will generate more sales this current year, than they have in prior years? Why yes or why...
-
Billy purchased a fleet of cars used in his business for $200,000 three years ago. On 7/1/Year 1 when his basis in the cars was $120,000 he sold them for $300,000. He incurred $5,000 of expenses in...
-
Use the following vapor pressures of propane given here to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P (Torr) T (K) 0.01114 100. 120 2.317...
-
Benzene(l) has a vapor pressure of 0.1269 bar at 298.15 K and an enthalpy of vaporization of 30.72 kJmol 1 . The C P,m of the vapor and liquid phases at that temperature are 82.4 and 136.0 J K 1 mol...
-
An air conditioner on a hot summer day removes 8 Btu/s of energy from a house at 70 F and pushes energy to the outside, which is at 88 F. The house has 30 000 lbm mass with an average specific heat...
-
What are automobile-guest statutes and why were they introduced?
-
What is the Learned Hand formula, and how does it help in assessing reasonableness?
-
What is the doctrine of respondeat superior?
-
How does the duty of a public entity compare to that of a private individual?
-
What conditions must be met to have an attractive nuisance? a. What characteristics of a child are taken into consideration when deciding whether the attractive-nuisance doctrine applies?
-
Find the frequency of the lowest vibrational mode of a diatomic molecule in terms of the parameters of the Morse potential, eq. Vstone () = De (1-e -r)* -1 -a(r-re
-
An Atomic Energy Commission nuclear facility was established in Hanford, Washington, in 1943. Over the years, a significant amount of strontium 90 and cesium 137 leaked into the Columbia River. In a...
-
Find the Fourier transform of the function f(x) = e |t| . Since this is an even function, you can use the one-sided cosine transform.
-
Construct a graph with the function f from the previous example and c 1 1 on the same graph. Let a = 1 for your graph. Comment on how well the partial sum with one term approximates the function.
-
Find the one-sided Fourier sine transform of the function ae bx .
-
Given the following Python code: x = 20 while x > 10: #do something x = x 1 How many times this loop will iterate. 10 11 20 20 0
-
The HTML program is fine I want help to figure out what's wrong with CSS that would not make my website look the same as the picture I provided. @charset "UTF-8"; /* CSS Document for CA3 */ body {...
-
Consider a twisted pair link of distance 2 km. It is required to compute the amount of received power Pr, assuming the transmit power Pt = 1 Watt and the cable attenuation is 20 dB/km.

Study smarter with the SolutionInn App


