Use the following data at 298.15 K to complete this problem: Calculate ÎH o R for a.
Question:
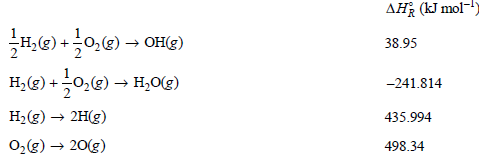
Calculate ΔHoR for
a. OH(g) †’ H(g) + O(g)
b. H2O(g) †’ 2H(g) + O(g)
c. H2O(g) †’ H(g) + OH(g)
Assuming ideal gas behavior, calculate ΔHoR and ΔUoR for all three reactions.
Transcribed Image Text:
AH (kJ mol- H,(g) +0,(e) → OH() H, (g) +0,(g) → H,0(g) 38.95 -241.814 H2(g) → 2H(g) 0,g) → 20(g) 435.994 498.34
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 65% (20 reviews)
a U o R H o R nRT 42822 kJ mol 1 8314 J mol 1 K 1 29815 K 42574 kJ m...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data Tables), calculate the standard enthalpy of formation of H 2 S(g) and of FeS 2 (s): AR(kJ mol) Fe(s) + 2H2S(g) ...
-
Calculate the standard enthalpy of formation of FeS 2 (s) at 600. °C from the following data at 298.15 K. Assume that the heat capacities are independent of temperature. You are also given that...
-
From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of Fe 2 O 3 (s): A(kJ mol) Fe,0;(s) + 3C(graphite) 2Fe(s) + 3cO(g) FeO(s) + C(graphite) Fe(s) + CO(g)...
-
The registers values in AX and BX can be exchanged using stack operations that are: Select one: a. PUSH bx PUSH ax POP bx POP AX b. none C. PUSH ax PUSH bx POP ax POP bx d. PUSH ax POP ax PUSH bx POP...
-
The average age of vehicles registered in the United States is 96 months. Assume the population is normally distributed with a standard deviation of 15 months. Find the probability that the mean age...
-
To suck lemonade of density 1000 kg/m3 up a straw to a maximum height of 4.0 cm, what minimum gauge pressure (in atmospheres) must you produce in your lungs?
-
(a) Show that Eqs. 8.47 reduce to Eqs. 8.46 when you consider a system of just one object. (b) Follow the procedure used to get from Eq. 8.21 to Eq. 8.24 for a system of many interacting objects....
-
Cougar, Inc., is a calendar year S corporation. Cougars Form 1120S shows nonseparately stated ordinary income of $80,000 for the year. Johnny owns 40% of the Cougar stock throughout the year. The...
-
Operating systems and application programs play vital role in our daily usage of computers. Differentiate between an operating system and an application program give examples each.
-
Do interruptions while you are working reduce your productivity? According to a University of California-Irvine study, businesspeople are interrupted at the rate of approximately 5 times per hour...
-
What is process integration? What does it have to do with the management of supply chains?
-
What is a dabbawala ?
-
Determine the reactions at the supports A and B. 700 lb/ft 20 ft 500 lb/ft B 30 ft 48 ft- 48 ft-
-
Comparative Analysis Case adidas and Puma The financial statements of adidas (DEU) and Puma (DEU) are presented in Appendices B and C, respectively. The complete annual reports, including the notes...
-
To protect American jobs, the U.S. government may decide to cut U.S. imports of bulldozers by 60 percent. It could do so by either (a) imposing a tariff high enough to cut bulldozer imports by 60...
-
Suppose that the United States currently imports 1.0 million pairs of shoes from China at \($20\) each. With a 50 percent tariff, the consumer price in the United States is \($30\). The price of...
-
Develop a survey instrument to measure attitudes toward jogging. Have 10 people complete the survey and then report your findings. How could these findings be used by your local running club to...
-
Assume that the Sharpe-Lintner CAPM holds, so the mean-variance efficient frontier consists of combinations of Treasury bills and the market portfolio. Nonetheless, some households make the mistake...
-
Construct bar charts showing the kinetic energy and potential energy of the projectile in Figure 6.16. Show these energies when the projectile has just left the ground, when it is at the highest...
-
The Pletcher Transportation Company uses a responsibility reporting system to measure the performance of its three investment centers: Planes, Taxis, and Limos. Segment performance is measured using...
-
Figure 6.40 is the phase diagram for silver and tin. Label the regions, and describe what will be observed when liquids of compositions a and bare cooled to 200 K.
-
Indicate on the phase diagram in Fig. 6.42 the feature that denotes incongruent melting. What is the composition of the eutectic mixture and at what temperature does it melt?
-
Sketch the cooling curves for the isopleths a and b in Fig. 6.42
-
If the local professional basketball team, the Sneakers, wins today's game, they have a 3 chance of winning their next game. If they lose this game, they have a chance of winning their next game. a)...
-
There is a function f of the form .12 f(x) = ax + x 13 for which f(0.1) = 6.06 10 and (0.9) = 0.03577. Determine a and B, and assess the sensitivity of these parameters to slight changes in the...
-
The order states: Give antihistamine elixir 2.5mg/kg/dose PO q4h. The patient weighs 143lbs. The drug is available as Antihistamine Elixir 200mg in every 8mL. Answer each of the following questions. ...

Study smarter with the SolutionInn App


