Use the vapor pressures for hexane given in the following table to estimate the temperature and pressure
Question:
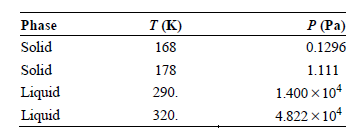
Transcribed Image Text:
Phase T (K) 168 P (Pa) 0.1296 Solid Solid Liquid Liquid 178 1.111 1.400 x 104 4.822 x 104 290. 320.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
To calculate the triple point temperature take subli...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use the vapor pressures for tetrachloromethane given in the following table to estimate the temperature and pressure of the triple point and also the enthalpies of fusion, vaporization, and...
-
Use the vapor pressures of ice given here to calculate the enthalpy of sublimation using a graphical method or a least squares fitting routine. T (K) P (Torr) 200. 0.1676 210. 0.7233 2.732 220. 230....
-
Use the vapor pressures of SO 2 (l) given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. T (K) 190. P (Pa) T (K) 230. P...
-
Midland Corporation has a net income of $19 million and 4 million shares outstanding. Its common stock is currently selling for $48 per share. Midland plans to sell common stock to set up a major new...
-
In 2014, Toyota faced significant sanctions in the case of Toyota's Recall Problems. Summarize those sanctions and comment on whether you believe they were minimal, adequate, or excessive.
-
An economist has predicted that there will be a 7 percent per year inflation of prices during the next 10 years. If this prediction proves to be correct, an item that presently sells for \(\$ 10\)...
-
Does Early Language Reduce Tantrums? A recent headline reads "Early Language Skills Reduce Preschool Tantrums, Study Finds," and the article offers a potential explanation for this: "Verbalizing...
-
Lance Armstrong won the Tour de France seven consecutive years (1999-2005). The following table gives the winning times, distances, speeds, and margin of victory. (a) Determine the mean and median of...
-
ces On January 1, 2024, the Mason Manufacturing Company began construction of a building to be used as its office headquarters. The building was completed on September 30, 2025. Expenditures on the...
-
Lake Erie has a water volume of about 450 km 3 and a flow rate (in and out) of about 175 km 2 per year. If at some instant the lake has pollution concentration p = 0.04%, how long, approximately,...
-
Which two business concepts are most highly correlated? a. Risk and reward b. Risk and revenue c. Reward and investment d. Investment and revenue
-
Show the paths n o p q and a b c d e f of the PVT phase diagram of Figure 8.15 in the PT phase diagram of Figure 8.4. Figure 8.4 Figure 8.15 Critical - point Liquid Solid Triple point Gas Tm...
-
Consider the Business Application, Assessing Product Profitability for Products with Large Development Expenses and Long Product Lives. What estimates can the manager make that affect the reported...
-
Why is word of mouth more powerful than an advertised message?
-
Mobile has a diverse array of advertising options. What are some of the most popular, and how can each help visually and verbally express the key consumer benefit?
-
What are the pros and cons of Internet and social media marketing?
-
Why is customer service considered a must for an IMC campaign?
-
Why are more brands incorporating alternative media into their promotional mix?
-
The US total electrical power consumption in 2010 was 3.9 TkWh. Utilities try to maintain a capacity that is twice the average power consumption to allow for high demand on hot summer days. What...
-
Grace is training to be an airplane pilot and must complete five days of flying training in October with at least one day of rest between trainings. How many ways can Grace schedule her flying...
-
The Hückel secular equation for the hydrogen molecule is Determine the two orbital energies in terms of α and β. = 0.
-
Find the eigenvalues and eigenvectors of the matrix 10 1 1 1 0
-
Find the eigenvalues and eigenvectors of the matrix 111 1 1
-
Reflect on a group interview you've had: * Was there anything you struggled with in preparing for the peer interviews? * What would you have changed about your interview? What would you have kept the...
-
Please conduct comprehensive research on the status of Metaverse, Choose a company, an industry, a product, or a service, and Share the strategies being used in Metaverse to develop a competitive...
-
Renovation and Restoration of community Park project For Risk management plan External risk : Budget Fluctuations in local economy Environment disasters Internal Risk: Safety Not enough money, staff...

Study smarter with the SolutionInn App


