Using the SRK equation of state (or any other cubic equation of state) to determine a specific
Question:
Using the SRK equation of state (or any other cubic equation of state) to determine a specific volume from a specified temperature and pressure requires a trial-and-error calculation. Three computer-based approaches to solving this problem may be used: (1) spreadsheet programs such as Excel’s Solver; (2) mathematical packages such as Mathcad, Mathematica, Matlab, and Polymath; and (3) programming languages such as Fortran and C++. The goal of this problem is to use the first two approaches to determine V̂(L/mol) for CO2 at (i) 200 K and 6.8 atm; (ii) 250 K and 12.3 atm; (iii) 300 K and 6.8 atm; (iv) 300 K and 21.5 atm; and (v) 300 K and 50.0 atm. (a) Starting with Equation 5.3-8, derive the following equivalent expression for the SRK equation of state:
![]()
(b) Prepare a spreadsheet to take as inputs a species identifier (such as CO2 ), the critical temperature, critical pressure, Pitzer acentric factor, and the temperatures and pressures for which V^ is to be calculated, and to calculate V̂ using Equations 5.3-8 to 5.3-13 for each of the specified conditions. The spreadsheet should have the following structure:
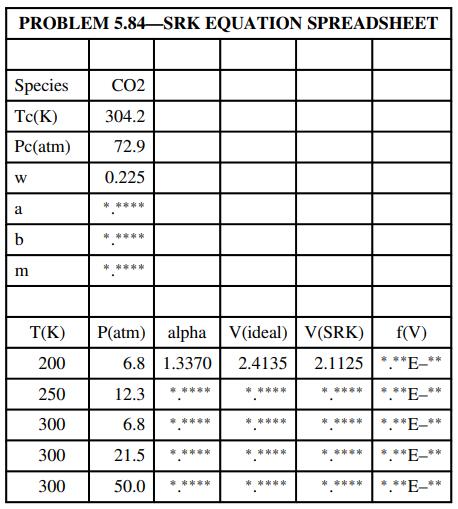
Single digits should appear in place of each asterisk shown on the table. Formulas should be entered into the row for 200 K and 6.8 atm and copied into the next four rows. The Goal Seek tool should be used to determine each V̂(SRK), starting with the ideal-gas value and varying the cell value to make f(V̂) as close as possible to zero.
(c) Use a root-finding procedure in a mathematical software package to determine V^ for each of the five conditions.
Equation 5.3-8 to 13
![]()
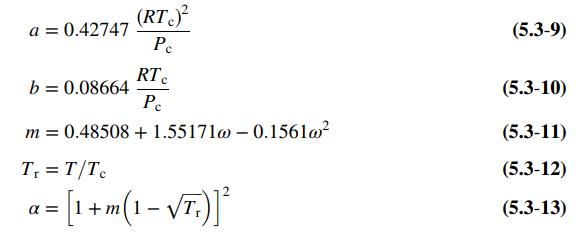
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





