Using your results from Problem P24.10, a. Calculate the s and p character of the water lone
Question:
a. Calculate the s and p character of the water lone pair hybrid orbitals
b. Show that the lone pair orbitals are orthogonal to each other and to the hybrid bonding orbitals.
In Problem 24.10
Derive two additional mutually orthogonal hybrid orbitals for the lone pairs on oxygen in H2O, each of which is orthogonal to and , by following these steps:
Starting with the following formulas for the lone pair orbitals
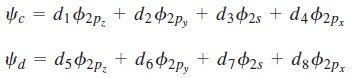
use symmetry conditions to determine d2 and d4 and to determine the ratio of d3 to d7 and of d4 to d8.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





