Why is the magnitude of the electron affinity for a given element smaller than the magnitude of
Question:
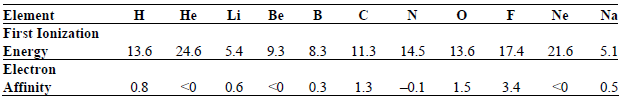
Transcribed Image Text:
в с Na Ne Не Li Be Element First Ionization н N 13.6 24.6 5.4 13.6 9.3 8.3 14.5 17.4 21.6 Energy Electron 11.3 5.1 0.3 <0 0.5 0.8 0.6 -0.1 3.4 1.5 Affinity <0 1.3 <0 3. 3. 3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Within Koopmans approximation in which it is assumed that the electron distribution in an atom is un...View the full answer

Answered By

Jehal Shah
I believe everyone should try to be strong at logic and have good reading habit. Because If you possess these two skills, no matter what difficult situation is, you will definitely find a perfect solution out of it. While logical ability gives you to understand complex problems and concepts quite easily, reading habit gives you an open mind and holistic approach to see much bigger picture.
So guys, I always try to explain any concept keeping these two points in my mind. So that you will never forget any more importantly get bored.
Last but not the least, I am finance enthusiast. Big fan of Warren buffet for long term focus investing approach. On the same side derivatives is the segment I possess expertise.
If you have any finacne related doubt, do reach me out.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Explain why the first ionization energy and electron affinity for F are larger than for O.
-
The first ionization energy and electron affinity of Ar are both positive values. (a) What is the significance of the positive value in each case? (b) What are the units of electron affinity?
-
Use electron configurations to explain the following observations: (a) The first ionization energy of phosphorus is greater than that of sulfur. (b) The electron affinity of nitrogen is lower (less...
-
discuss the benefits and challenges of the employee job performance evaluation process as it relates to the ratings of an individual. For example, if an employer uses a standard Likert Scale (1...
-
In Exercises 59 and 60, determine whether the statement is true or false. Justify your answer. 1. The Leaning Tower of Pisa is not vertical, but when you know the angle of elevation to the top of...
-
Davidson Hardware used the FIFO inventory method in 2012. Davidson plans to continue using the FIFO method in future years. Requirement 1. Which inventory principle is most relevant to Davidsons...
-
Choose an appropriate closed system and draw a bar diagram representing the energy conversions and transfers that occur during each process of Checkpoint 7.9: (a) a ball launching as the compressed...
-
You have been asked by the board of trustees of a local church to review its accounting procedures. As part of this review you have prepared the following comments about the collections made at...
-
A hiker and his dog have discovered a skeleton hidden deep in the woods. The medical examiner has identified this skeleton as female. What characteristics of the skeleton would help the examiner make...
-
Malo Inc. uses a fiscal year ending June 30. On May 29, Malo received a check for $3,900 from a business that leases parking spaces in Malo's parking garage. This payment was for the three-month...
-
The electron affinities of He, Be, and Ne are negative, meaning that the negative ion is less stable than the neutral atom. Explain why this is so for these three elements.
-
Calculate the position of the maximum in the radial distribution function for Li 2+ in its ground state using the wave function in P21.13.
-
A function of two variables that satisfies Laplace's Equation, Is said be harmonic. Show that the functions defined in Problems 1 and 2 are harmonic functions. 1. ((x, y) = x3 y - xy3 2. ((x, y) =...
-
P6. (6 pts) Consider a generator of a CRC scheme 1010011 (i.e., x6+x++x+1). The message is 00110011 10001010. Answer the following questions. a. Compute the CRC bits for this packet (ignoring all...
-
n enterprise elected to use the cost model to subsequently measure its land. The value of the land it holds is now impaired as it was purchased at a cost of $100,000, and it is now worth only...
-
What are the obligations incumbent upon a vessel to its state flag, encompassing the array of responsibilities and commitments it must uphold in accordance with maritime regulations and international...
-
Determine the risks and efficiencies of an updated computerized accounting information system. Include the following in your response: Impact on an organization's financial reporting Effect on...
-
The contribution amount paid to Daniella different to the SG contribution amount due to different of rate used. Which ethical principle and practice has been breached? Describe how it has been...
-
Figure 5.19 shows a pump partially submerged in oil (sg = 0.90) and supported by springs. If the total weight of the pump is 14.6 lb and the submerged volume is 40 in 3 , calculate the supporting...
-
You are maintaining a subsidiary ledger account for Police-Training Expenditures for 2013. The following columns are used: Inventory purchases are initially recorded as expenditures. Record the...
-
The osmotic pressure of solutions of polystyrene in toluene were measured at 25C and the pressure was expressed in terms of the height of the solvent of density 1.004 g cm 3 : Calculate the molar...
-
A water carbonating plant is available for use in the home and operates by providing carbon dioxide at 5.0 atm. Estimate the molar concentration of the soda water it produces.
-
What proportions of hexane and heptane should be mixed (a) By mole fraction, (b) By mass in order to achieve the greatest entropy of mixing?
-
a) Solve cos x = 2xy and cos xy = 2x to 5 decimal places with an initial guess of x0 = 0.5 and yo= 0.5 using proper method. (90 Point) ATTENTION: Please add a comment line to each line of code...
-
Write a program that will asks the user to input 15 students test score then store them in an array named "Score" your program should accomplish the followings: 1. Calculate and display the average....
-
4. What is clock synchronization? Synchronize the network given below when the server. advances 10 second using Berkeley algorithm. Server 2:35 2:00 2:20 1:55 Client 1 Client 2 Client 3

Study smarter with the SolutionInn App


