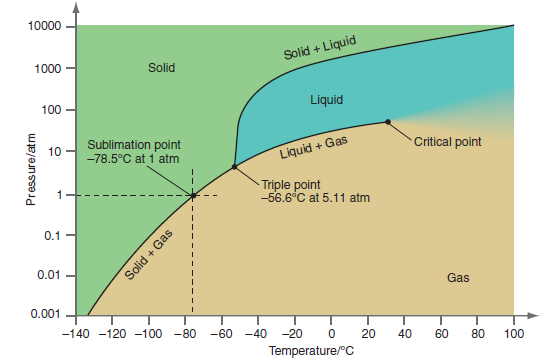
Within what range can you restrict the values of P and T if the following information is
Question:
a. As the temperature is increased, the solid is first converted to the liquid and subsequently to the gaseous state.
b. An interface delineating liquid and gaseous phases is observed throughout the pressure range between 6 and 65 atm.
c. Solid, liquid, and gas phases coexist at equilibrium.
d. Only a liquid phase is observed in the pressure range from 10. to 50. atm.
e. An increase in temperature from ˆ’80.° to 20.°C converts a solid to a gas with no intermediate liquid phase.
Figure 8.12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





