An early model of the atom, proposed by Rutherford after his discovery of the atomic nucleus, had
Question:
a. Show that the electric field strength inside this atom is
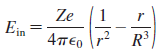
b. What is E at the surface of the atom? Is this the expected value? Explain.
c. A uranium atom has Z = 92 and R = 0.10 nm. What is the electric field strength at r = 1/2 R?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:





