Calculate the voltage of the following cell, in which KHP is potassium hydrogen phthalate, the monopotassium salt
Question:
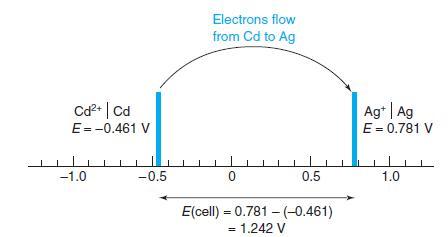
Calculate the voltage of the following cell, in which KHP is potassium hydrogen phthalate, the monopotassium salt of phthalic acid. By the reasoning in Figure 13-8, in which direction do electrons flow?
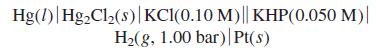
Figure 13-8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




