Make a graph of [Ag + ], [AgOH(aq)], [CN - ], and [HCN] as a function of
Question:
Make a graph of [Ag+], [AgOH(aq)], [CN-], and [HCN] as a function of pH in a saturated solution of AgCN.
Consider the following equilibria and do not consider activity coefficients. Find the pH if no buffer were added.
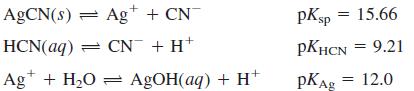
Transcribed Image Text:
AGCN(s) = Ag+ + CN pKsp = 15.66 HCN(aq) = CN + H+ PKHCN = 9.21 Ag* + H2O = AGOH(aq) + H pKAg = 12.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
To create a graph of the concentrations of Ag AgOHaq CN and HCN as a function of pH in a saturated solution of AgCN we need to consider the given equi...View the full answer

Answered By

M Ali Hasan
As a student tutor i am very proud to helping other students and sharing my knowledge to the students community I am very happy to work in solutioninn.com
As a tutor if they provide me the job I m very glad to provide the quality education and quick step by step answers to all the students
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Consider the following equilibria in aqueous solution: (a) Calculate the numerical value of the equilibrium constant for the reaction (b) Calculate the concentration of AgCl(aq) in equilibrium with...
-
Find the concentrations of Ag+(aq), NH3(aq), and [Ag(NH3)2]+(aq) at equilibrium when 0.10 mol Ag+(aq) and 0.10 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for the...
-
The formation constant for the reaction Ag+ + 2NH3 [Ag(NH3)2]+ is 1.5 Ã 107 and that for the reaction Ag+ + 2CN- [Ag(CN)2]- is 1.0 Ã 1021 at 25°C (see Table 16.3). Calculate the...
-
Using atomic weight, crystal structure, and atomic radius data tabulated inside the front cover of the book, compute the theoretical densities of aluminum (Al), nickel (Ni), magnesium (Mg), and...
-
Independent samples of sizes nA = 2 and nB = 2 are taken from two continuous populations. (a) Enumerate all possible collections of ranks associated with population A. Also attach probabilities to...
-
You are investigating the service quality of restaurants. You are collecting primary data through interviews and observation. Your task is to go to a restaurant and collect descriptive observational...
-
We live in a global economy, and many different external activities have an impact on the business. On what basis would we narrow the scope of this book to de-emphasize tax law, currency exchange,...
-
At June 30, 2015, the end of its most recent fiscal year, Red River Computer Consultants Ltd.'s post-closing trial balance was as follows: The company underwent a major expansion in July. New staff...
-
Find the vertical asymptotes, if any, and the values of x corresponding to holes, if any, of the graph of the rational function.. f(x)= x-6 X-9x+18
-
Mar, a U.S. firm, purchased equipment for 400,000 British pounds from The on December 16, 2016. The terms were n/30, payable in British pounds. On December 16, 2016, Mar also entered into a 30-day...
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
Difference plot. A solution containing 3.96 mmol acetic acid plus 0.484 mmol HCI in 200 mL, of 0.10 M KC1 was titrated with 0.490 5 M NaOH to measure K. for acetic acid. (a) Write expressions for the...
-
The comparative balance sheet of Harris Industries Inc. at December 31, 2014 and 2013, is as follows: An examination of the income statement and the accounting records revealed the following...
-
It is not possible to copy/paste elements from one floor to another and have them line up (with the original objects). A) True B) False
-
Fill in the blank field in this text: While using the Roof tool, you can use the[1]__________ tool from the Ribbon to fill in the missing segments to close the perimeter.
-
Fill in the blank field in this text: Use the [1]_______________command to create a reverse image.
-
Revit provides several different styles of vanity cabinets for placement. A) True B) False
-
When creating a roof using the create roof by footprint option, you need to create a closed perimeter. A) True B) False
-
What are the two basic categories of current liabilities? Give an example of each.
-
San Carlos Bank and Trust Company uses a credit-scoring system to evaluate most consumer loans that amount to more than $2,500. The key factors used in its scoring system are found at the conclusion...
-
What is selected reaction monitoring? Why is it also called MS/MS? Why does it improve the signal/noise ratio for a particular analyte?
-
(a) To detect the drug ibuprofen by liquid chromatography/ mass spectrometry, would you choose the positive or negative ion mode for the spectrometer? Would you choose acidic or neutral...
-
An electrospray/transmission quadrupole mass spectrum of the -chain of hemoglobin from acidic solution exhibits nine peaks corresponding to M n+ n . Find the charge, n, for peaks A-I. Calculate the...
-
Tasks : Your business memo should address the following scenario/prompt You business memo will be addressed to all employees at your company. It will announce a new policy of "paid time off" based on...
-
THESIS: MY DREAM IS TO BECOME A BSN NURSE Instructions The following are the guidelines for creating your informative speech PowerPoint for the presentation: Slide 1: Title Slide: Include the title,...
-
For each problem: a) Find the eigenvalues and eigenvectors of A "by hand", writing and solving the characteristic equation. (You do not need to type this part, just write it neatly, if you like). b)...

Study smarter with the SolutionInn App


