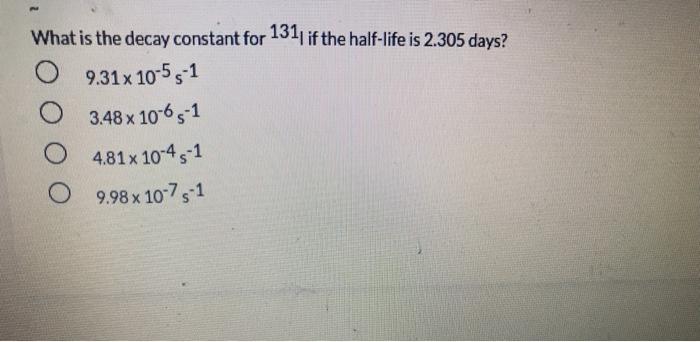What is the decay constant for 131 if the half-life is 2.305 days? 9.31x 10-5 s1...
Fantastic news! We've Found the answer you've been seeking!
Question:
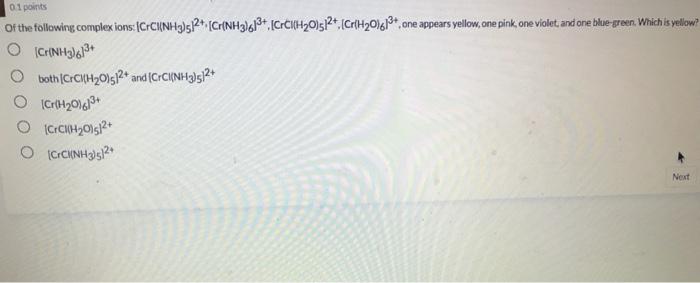
![Colen) C1_]* has a coordination number of and a geometry of O None of these complexes has coordination number 6. 4, square pl](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2021/08/610b328979b39_785610b32893e049.jpg)

Transcribed Image Text:
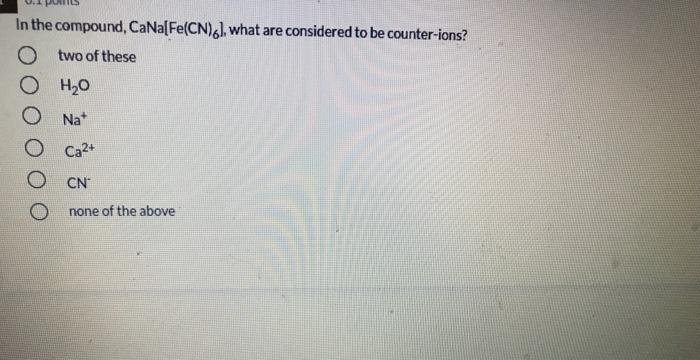
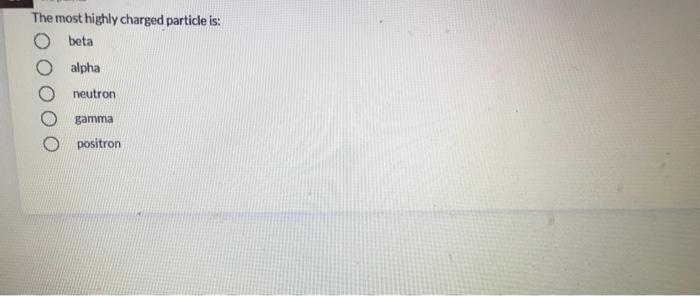
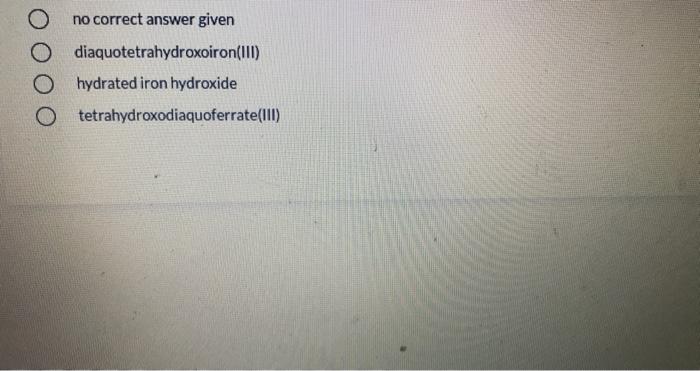
What is the decay constant for 131 if the half-life is 2.305 days? 9.31x 10-5 s1 3.48 x 1065-1 4.81x 10-45-1 9.98 x 10 75-1 01 points Of the following complex ions: [CrC(NH3)5R+,(Cr{NH3)6*,ICrCIH2Ols2t,(CrH2016*, one appears yellow, one pink, one violet, and one blue-green. Which is yellow? both (CFCIH20)s2* and (CHCI{NH9I5?+ Next In the compound, CaNa[Fe(CN),), what are considered to be counter-ions? two of these O H20 O Na* O Ca2+ O CN none of the above [Co(en),Cl,]* has a coordination number of and a geometry of O None of these complexes has coordination number 6. 4, square planar 6, square planar 4, tetrahedron 6, octahedron two of these The most highly charged particle is: beta alpha neutron gamma positron no correct answer given diaquotetrahydroxoiron(II) hydrated iron hydroxide O tetrahydroxodiaquoferrate(lII) The beta decay of 24 F produces an isotope of 10 Al Na Ne O Mg What is the decay constant for 131 if the half-life is 2.305 days? 9.31x 10-5 s1 3.48 x 1065-1 4.81x 10-45-1 9.98 x 10 75-1 01 points Of the following complex ions: [CrC(NH3)5R+,(Cr{NH3)6*,ICrCIH2Ols2t,(CrH2016*, one appears yellow, one pink, one violet, and one blue-green. Which is yellow? both (CFCIH20)s2* and (CHCI{NH9I5?+ Next In the compound, CaNa[Fe(CN),), what are considered to be counter-ions? two of these O H20 O Na* O Ca2+ O CN none of the above [Co(en),Cl,]* has a coordination number of and a geometry of O None of these complexes has coordination number 6. 4, square planar 6, square planar 4, tetrahedron 6, octahedron two of these The most highly charged particle is: beta alpha neutron gamma positron no correct answer given diaquotetrahydroxoiron(II) hydrated iron hydroxide O tetrahydroxodiaquoferrate(lII) The beta decay of 24 F produces an isotope of 10 Al Na Ne O Mg
Expert Answer:

Related Book For 

Posted Date:
Students also viewed these chemistry questions
-
A number of indirect compensation benefits are considered to be taxable benefits to the employee. What advantage can the employer and/or the employee gain by including such benefits as part of a...
-
In India, independent auditors are considered to be "watchdogs," not "bloodhounds." How, if at all, does that concept of the auditor's role differ from the prevailing concept of the independent...
-
(a) What is the decay constant of 238/92U whose half-life is 4.5 X 109 yr? (b) The decay constant of a given nucleus is 8.2 X 10 5 s 1. What is its half-life?
-
Find the indefinite integrals (a) sin 3x cos 5x (b) cos 7x cos 5x (c) sin 2 x (d) cos 2 x (e) cosh 2 x (f) sinh(5x + 1)
-
1. What kind of decisions has chief executive Ben Verwaayen been making at Alcatel-Lucent? 2. To what extent are these decisions subject to risk and uncertainty? 3. To what extent do you think...
-
A 12.0-kg shell is launched at an angle of 55.0 above the horizontal with an initial speed of ISO m/s. When it is at its highest point the shell exploded into two fragments, one three times heavier...
-
The current loop in Figure P28.34 lies in the \(x y\) plane. For each of the Amperrian paths (a)-(e), is the line integral of the magnetic field positive, negative, or zero? Data from Figure P28.34...
-
Eagle Manufacturing supplies engine parts to Cherokee Cycle Company, a major U.S manufacturer of motorcycles. Like all of Cherokees suppliers, Eagle has always added a healthy profit margin to its...
-
All work must be shown on every facet of the solution. This includes a timeline with cashflows, timing and rates clearly laid out and formulas (in pure form, not in the calculator or excel form). A...
-
Danny Imasuen is a 37-year-old student working in Quebec. His wages for the current weekly pay period are $580.00. The employer pays $22.00 for life insurance premiums and $190.00 for group medical...
-
(a) CH3CH2 OH 10C
-
The owner of Metro Sports wishes to determine the number of advertisements to be placed in the selected three monthly magazines A, B and C. His objective is to advertise in such a way that total...
-
Suppose you are on a plane from Dallas, Texas, to Santiago, Chile. On the way there, you realize something amazing. You have just experienced the longest day of the year in the Northern Hemisphere...
-
Postmodern approaches to knowledge are said to be different from modern ones. How exactly are they different? What are the key components of each tradition of thought? What are the concrete political...
-
Reinforcing bars are nominated on drawings using a particular convention. Explain this with an example ?
-
Most studies of mutual fund performance conclude that managers cannot consistently exceed the average return in the stock market as a whole. Why might you expect this result? What does it imply about...
-
1 of these Short Answer Questions requires you to refer to the state/territory-specific requirements, such as legislation and regulations of your state/territory. For your assessor's reference, put...
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
Consider Fig. 29-10. As the last phosphate group approaches and then bonds to the ADP molecule, which of the following is true? Choose all that apply. (a) The phosphate group is first repelled and...
-
Alpha particles (charge q = +2e, mass m = 6.6 10-27 kg) move at 1.6 106 m/s. What magnetic field strength would be required to bend them into a circular path of radius r = 0.14 m?
-
You are trying to push your stalled car. Although you apply a horizontal force of 400 N to the car, it doesn't budge, and neither do you. Which force(s) must also have a magnitude of 400 N? (a) The...
-
We are given three observations on binary choice with \(y_{1}=1, y_{2}=1, y_{3}=0\). Consider a logit model with only an intercept, \(P(y=1)=\Lambda\left(\gamma_{1} ight)\), where...
-
In Example 16.3, we illustrate the calculation of the likelihood function for the probit model in a small example. In this exercise, we will repeat that example using logit instead of probit. The...
-
In this exercise, we generalize the results in Exercise 16.5. Consider a logit model with only an intercept, \(P(y=1)=\Lambda\left(\gamma_{1} ight)\), where \(\Lambda(\bullet)\) is the logistic \(c d...

Study smarter with the SolutionInn App