A dialyzer is to be used to separate 300 L/h of an aqueous solution containing 0.1-M NaCl
Question:
A dialyzer is to be used to separate 300 L/h of an aqueous solution containing 0.1-M NaCl and 0.2-M HCl. Laboratory experiments with the microporous membrane to be used give the following values for the overall mass-transfer coefficient Ki in (14-79) for a log-mean concentration-driving force:
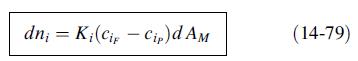
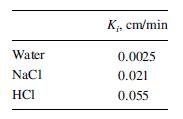
Determine the membrane area in m2 for 90, 95, and 98% transfer of HCl to the diffusate. For each case, determine the complete material balance in kmol/h for a sweep of 300 L/h. will be the same in each stage and the expected current efficiency is 90%. The applied voltage for the first stage is 220 V. Each cell pair has an area of 1,160 cm2. Calculate the current density in mA/cm2, the current in A, and the power in kW for the first stage. Reference: Mason, E.A., and T.A. Kirkham, C.E.P. Symp. Ser., 55 (24), 173–189 (1959).
Step by Step Answer:

Separation Process Principles Chemical And Biochemical Principles
ISBN: 9780470481837
3rd Edition
Authors: By J. D. Seader, Ernest J. Henley, D. Keith Roper





