The pH of a liquid is a measure of its acidity or alkalinity. Pure water has a
Question:
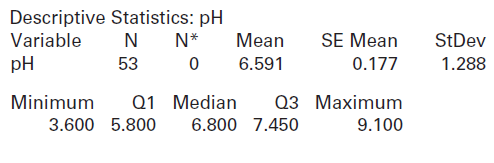
(a) How many lakes are included in the dataset? What is the mean pH value? What is the standard deviation?
(b) Use the descriptive statistics above to conduct a hypothesis test to determine whether there is evidence that average pH in Florida lakes is different from the neutral value of 7. Show all details of the test and use a 5% significance level. If there is evidence that it is not neutral, does the mean appear to be more acidic or more alkaline?
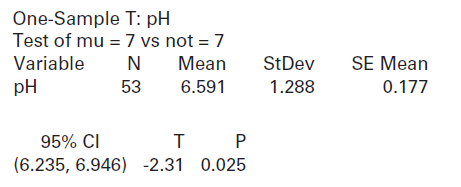
(c) Compare the test statistic and p-value you found in part (b) to the computer output below for the same data:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Statistics Unlocking The Power Of Data
ISBN: 9780470601877
1st Edition
Authors: Robin H. Lock, Patti Frazer Lock, Kari Lock Morgan, Eric F. Lock, Dennis F. Lock
Question Posted:





