(a) When a mixture containing one mole each of the three dimethylbenzenes (o-, m-, and p-xylene) is...
Question:
(a) When a mixture containing one mole each of the three dimethylbenzenes (o-, m-, and p-xylene) is treated with one mole of chlorine in the presence of a Lewis acid catalyst, one of the three hydrocarbons is monochlorinated in 100% yield, whereas the other two remain completely unreacted. Which isomer reacts? Explain the differences in reactivity.
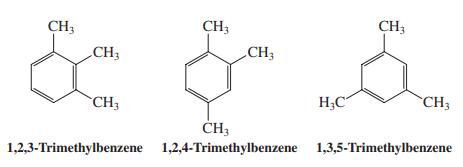
(b) The same experiment carried out on a mixture of the following three trimethylbenzenes gives a similar outcome. Answer the questions posed in (a) for this mixture of compounds.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





