Compare the addition of H1 to 1,3-pentadiene and 1,4-pentadiene (see Problem 46). Draw the structures of the
Question:
Compare the addition of H1 to 1,3-pentadiene and 1,4-pentadiene (see Problem 46). Draw the structures of the products. Draw a qualitative reaction profile showing both dienes and both proton addition products on the same graph. Which diene adds the proton faster? Which one gives the more stable product?
Data From Problem 46
Compare the allylic bromination reactions of 1,3-pentadiene and 1,4-pentadiene. Which should be faster? Which is more energetically favorable? How do the product mixtures compare?
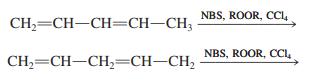
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





