-Dicarbonyl compounds condense with aldehydes and ketones that do not undergo self-aldol reaction. The products are ,-unsaturated...
Question:
β-Dicarbonyl compounds condense with aldehydes and ketones that do not undergo self-aldol reaction. The products are α,β-unsaturated dicarbonyl compounds, and the process goes by the colorful name of Knoevenagel condensation. (a) An example of a Knoevenagel condensation is given below. Propose a mechanism.
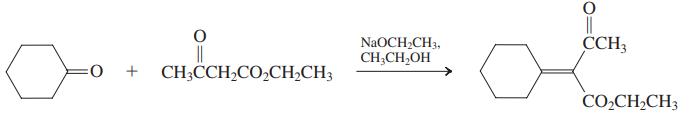
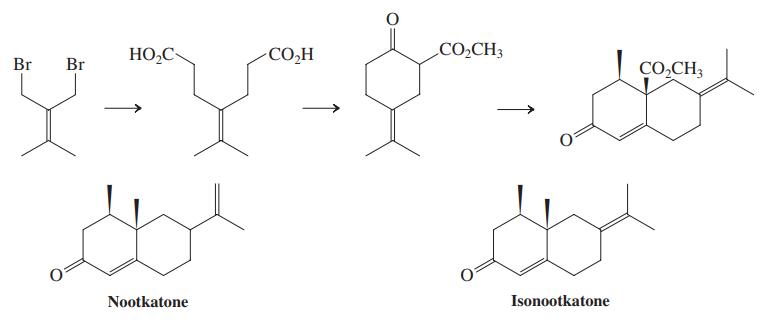
(c) The diester shown in the margin is the starting material for the dibromide used in the synthesis of isonootkatone (Problem 45). Suggest a preparation of this diester using the Knoevenagel condensation. Propose a sequence to convert it into the dibromide of Problem 45.
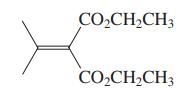
Problems 45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





