Explain the fact that, although hemiacetal formation between methanol and cyclohexanone is thermodynamically disfavored, addition of methanol
Question:
Explain the fact that, although hemiacetal formation between methanol and cyclohexanone is thermodynamically disfavored, addition of methanol to cyclopropanone goes essentially to completion:
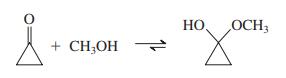
Transcribed Image Text:
HO НО OCH3 + CH;OH 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Hemiacetal formation bw methanol and cyclohexanone is thermodynamically unfavorable This can be ...View the full answer

Answered By

Bharat sharma
• Strong theoretical and practical knowledge of chemistry
• In-depth knowledge of chemistry and ability to teach students
• Ability to develop and execute lessons plans
• Skilled in a variety style of teaching methods and techniques
• Experience of conducting research and documenting results
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
How can you explain the fact that franc-i -hromo-2-methylcyclohcxane yields the non-Zaitsev?s elimination product 3-methylcyclohexene on treatment with base? H C Br trans-1-Bromo-2-methylcyclohexane...
-
How might Kekule explain the fact that there is only one dibromobenzene with the bromines on adjacent carbon atoms, even though we can draw two different structures, with either a double or a single...
-
The Expectations Theory of the Term Structure cannot explain the fact that treasury yield curves are generally upward sloping, and become downward sloping only infrequently. How can the Liquidity...
-
Match the following ratios with the appropriate formula. Ratio or Rate Formula a. Income from operations Interest expense Acid-test Total liabilities Stockholders' equity Current b. Net income-...
-
Consider the two-period com economy of the text. Suppose that initially the equilibrium has a zero trade deficit. Now, increase the interest rate. Show how the Fisher diagram changes. Will trade...
-
Discuss methods used by the U.S. government to regulate hedge funds.
-
Consider the following cash flow profile and assume MARR is 10 percent/year. a. What does Descartes' rule of signs tell us about the IRR(s) of this project? b. What does Norstrom's criterion tell us...
-
Wie Company has been operating for just 2 years, producing specialty golf equipment for women golfers. To date, the company has been able to finance its successful operations with investments from...
-
What is JDBC ? Explain the role of Driver in JDBC. What is the purpose Class.forName method ? What is the advantage of PreparedStatement over Statement ? What is the use of CallableStatement ? Name...
-
Amboy Specialty Foods: A Cheesy Variance Investigation In 1940, a retired U.S. Marine Colonel established Amboy Specialty Foods to provide canned cheese sauce as meal rations for the United States...
-
Propose efficient syntheses of each of the following molecules, beginning with the indicated starting materials. (a) (b) (c) from H;C HO, H
-
The rate of the reaction of NH2OH with aldehydes and ketones is very sensitive to pH. It is very low in solutions more acidic than pH 2 or more basic than pH 7. It is highest in moderately acidic...
-
On March 1, the Garner Corporation borrowed $75,000 from the First Bank of Midlothian on a one-year, 5 percent note. Required: If the company keeps its records on a calendar year, what adjusting...
-
Explanation of how Institute of Certified Bookkeepers is relevant to administration of subsidiary accounts and ledgers?
-
What do we call the movement of materials and components around a business so that the various value adding operations can be performed?
-
A hoop is rolling without slipping along a horizontal surface with a forward speed of 6.42 m/s when it starts up a ramp that makes an angle of 13.37 with the horizontal. What is the linear speed of...
-
Identify features of the temporal method. Multiple select question. The temporal method converts the recording currency to the functional currency. The temporal method remeasures the financial...
-
Bradenton Inc. is working on its Cash Budget for next year. Bradenton would like to end the first quarter with an Ending Cash Balance of $100,000. Bradenton is assuming the following for the first...
-
Helena has the following long-term capital gains and losses for 2014: $65,000 28% gain, $53,000 28% loss, $28,000 25% gain, and $24,000 0%/15%/20% loss. She also has a $33,000 short-term loss and a...
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
What is the hybridization at the N and each C in this molecule? Indicate the type of bond and the orbitals that are overlapping to form it for each of the designated bonds (for example, ?CSP3 + H1s)....
-
Consider hydrogen cyanide, H C N. (a) What is the hybridization at the N at the C? (b) What are the types of the three CN bonds? What orbitals overlapping to form them? (c) In what type of orbital...
-
What is the hybridization at each C in this molecule? Indicate the type of bond and the orbital's that are overlapping to form it for each of the designated bonds? ITT H=C=C=C=C_C7H (both) H tall...
-
How can bash shell scripting improve resource utilization and process management in Unix systems?
-
A solid sphere that is uniformly positively charged produces an electric field. Assume no other objects are around. What is the magnitude of the electric field a distance r from the center of the...
-
Why is potential difference important in x - ray production?

Study smarter with the SolutionInn App


