Ketoses show positive Fehlings and Tollenss tests not only by oxidation to -dicarbonyl compounds, but through a
Question:
Ketoses show positive Fehling’s and Tollens’s tests not only by oxidation to α-dicarbonyl compounds, but through a second process: Ketoses isomerize to aldoses in the presence of base. The aldose then undergoes oxidation by the Fehling’s or Tollens’s solution. Using any ketose in Figure 24-2, propose a base-catalyzed mechanistic pathway to the corresponding aldose.
Figure 24-2
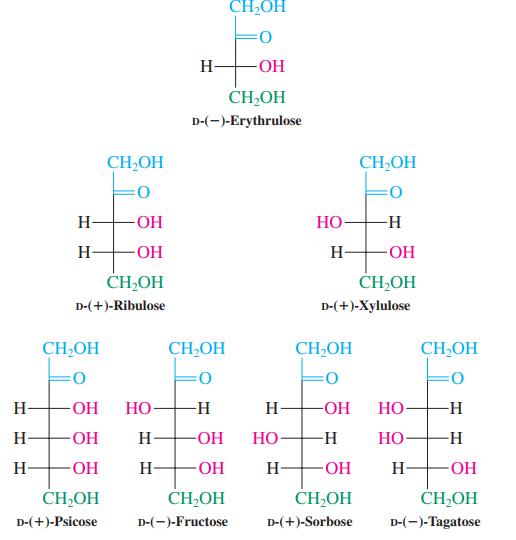
Transcribed Image Text:
CH,OH H- -ОН CH,OH D-(-)-Erythrulose CH,OH CH,OH H FOH HO H H- H FOH CH-OH CH,OH D-(+)-Ribulose D-(+)-Хylulose CH,OH CH,OH CH;OH CH,OH O: H- O- Но- -H H- -ОН Но- -H H- H- HO- Но- -H- Но- -H- H OH H- -ОН H- -ОН H- OH CH2OH CH,OH CH,OH ČH,OH D-(+)-Psicose D-(-)-Fructose D-(+)-Sorbose D-(-)-Тagatose
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The interconversion of hydroxy Aldehydes into hydroxy ketones in the basic medi...View the full answer

Answered By

JAIPRATAP SINGH SONU
My expertise is chemistry, mostly organic chemistry. This involves synthesizing, characterizing, and identifying organic molecules. I'll guide you towards best resources for learning key concepts that'll make you self-sufficient. You can expect the following : 1) Use of standard text books. 2) Use of peer- Reviewed Journals. 3) Use of reputed institute's resources. 4) Timely help for assignments and projects. 5) Help in learning key concepts. The solutions would be provided in pdf or word format within promised time, without any delay. There is a difference between solutions provided for Home-Work problems and solutions for extra credit assignments. Higher quality solutions with references would cost more but their sterling quality would be guaranteed, on the face of it. You would be able to trace back, the resources used for solutions provided to you.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
A hydrocarbon A, C9H12, is treated with A-bromosuccinimide in CCl4 in the presence of peroxides to give a compound B, C9H11Br. Compound B undergoes rapid solvolysis in aqueous acetone to give an...
-
Show how the following experimental evidence can be used to deduce the structure of lactose (Section 22.12D): 1. Acid hydrolysis of lactose (C12H22O11) gives equimolar quantities of D-glucose and...
-
A disaccharide forms a silver mirror with Tollens reagent and is hydrolyzed by a When the disaccharide is treated with excess methyl iodide in the presence of (a reaction that converts all the OH...
-
X-Tech Inc. produces specialized bolts for the aerospace industry. The operating cost of producing a single bolt is $2. The company currently sells the bolts for $6/unit. Each time the company...
-
Use the data in INTQRT.RAW for this exercise. (i) Using the data from all but the last four years (16 quarters), estimate an AR(1) model for r6t. (We use the difference because it appears that r6t...
-
Consolidated financial statements are typically prepared when one company has a controlling interest in another unless a. The subsidiary is a finance company. b. The fiscal year-ends of the two...
-
The Howell Corporation has the following account balances (in millions): Prepare an income statement and a supporting schedule of cost of goods manufactured for the year ended December 31, 2017. (For...
-
Dorex, Inc., presented the following comparative income statements for 2009, 2008, and 2007: Required a. Calculate the following for 2009, 2008, and 2007: 1. Net profit margin 2. Return on assets 3....
-
Explain the ways in which the firms and industries that led cities' recoveries from de-industrialization are similar and different from the firms and industries that led the industrial revolution....
-
Sponsored search, or pay-per-click search advertisements, are a popular form of internet advertising. These ads show up along other search results when a consumers searches for various terms on...
-
Draw the most stable pyranose conformation of each of the following sugars. (a) -D-Arabinose; (b) -D-galactose; (c) -D-mannose; (d) -D-idose.
-
What are the products (and their ratios) of periodic acid cleavage of each of the following substances: (a) 1,3-dihydroxyacetone; (b) rhamnose (Problem 34); (c) glucitol. Problem 34 H- OH H OH H-...
-
What are the issues that arise when two people try to share leadership? What is likely to contribute to the success or failure of such a partnership?
-
2- Please formulate the parametric mathematical modeling for the three-layer supply chain represented below: Supplier 1 Supplier 2 Supplier 3 2 D S 6 Supplier 4 7 4 Manufacturer 1 Manufacturer 1...
-
Part 2 Write a 500-750 word rationale and reflection on developing instruction. Include the following: Explain how your instructional choices in the lesson plan meet the needs of all students, and...
-
Charlie Chaplin, the 'silent comedian,' made in 1940. It is considered one of the great speeches of all time: How does listening to this speech make you feel? What do you think makes it an effective...
-
1) Why would I put these "hat Walk On By" and "T's a While Industry" on the course the same wock? How are hay under? How we Poy ales i? 7) How is Brent Staples' tone in "Just Walk On By" different...
-
BCC is completely acceptable in business/professional work. Do you use BCC? If so, for what reasons? Does it really matter if you use BCC or not, as for email you can just simply forward the email...
-
On July 1, 2010, Rex purchases a new automobile for $40,000. He uses the car 80% for business and drives the car as follows: 8,000 miles in 2010, 19,000 miles in 2011, 20,000 miles in 2012, and...
-
What are bounds and what do companies do with them?
-
Show the major product(s) from reaction of the following substances with (i) CH3CH2Cl, AlCl3 and (ii) HNO3,H2SO4 (b) (a)
-
The herbicide oxyfluorfen can be prepared by reaction between a phenol and an aryl fluoride. Propose amechanism. FC. F3C. "CH-CH NO2 "CH-CH NO2 yfluorfen
-
Treatment of p-bromotoluene with NaOH at 300 oC yields mixture of two products but treatment of m-bromotoluene with NaOH yields a mixture of three products. Explain.
-
You have just been asked to take over recording the warranties for the company's product. Currently, your company is using the cash basis for recording warranty expenses. Should that method continue...
-
What is the difference between National Health Accounts and Health Consumption Expenditures?
-
Sunfish & Company sold additional shares of common stock to an investor for $9,000. What would be the effect of this transaction on the accounting equation? Sunfish & Company sold additional shares...

Study smarter with the SolutionInn App


