Quaternary ammonium salts catalyze reactions between species dissolved in two immiscible phases, a phenomenon called phase-transfer catalysis.
Question:
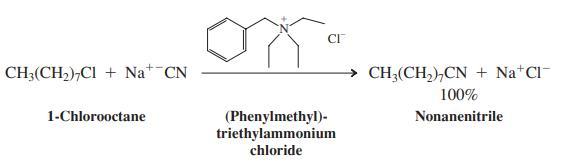
Quaternary ammonium salts catalyze reactions between species dissolved in two immiscible phases, a phenomenon called phase-transfer catalysis. For example, heating a mixture of 1-chlorooctane dissolved in decane with aqueous sodium cyanide shows no sign of the SN2 product, nonanenitrile. On the other hand, addition of a small amount of (phenylmethyl)triethylammonium chloride results in a rapid, quantitative reaction.
As a team, discuss possible answers to the following questions:
(a) What is the solubility of the catalyst in the two solvents?
(b) Why is the SN2 reaction so slow without catalyst?
(c) How does the ammonium salt facilitate the reaction?
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore




