Tertiary amines add readily as nucleophiles to carbonyl compounds, but, lacking a hydrogen on nitrogen, they cannot
Question:
Tertiary amines add readily as nucleophiles to carbonyl compounds, but, lacking a hydrogen on nitrogen, they cannot deprotonate to give a stable product. Instead, their addition gives an intermediate that is highly reactive toward other nucleophiles. Thus, tertiary amines are occasionally used as catalysts for the addition of weak nucleophiles to carboxylic acid derivatives.
(a) Fill in the missing structures in the scheme below.
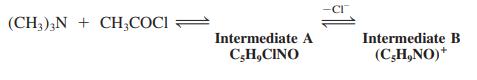
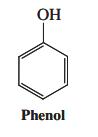
(b) Intermediate B readily enters into reactions with weak nucleophiles such as phenol (margin). Formulate the mechanism for this process and give the structure of the product.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:





