The general equation for the Baeyer-Villiger oxidation (see p. 808) begins with a reaction between a ketone
Question:
The general equation for the Baeyer-Villiger oxidation (see p. 808) begins with a reaction between a ketone and a peroxycarboxylic acid to form a peroxy analog of a hemiacetal. Formulate a detailed mechanism for this process.
Data From Page 808
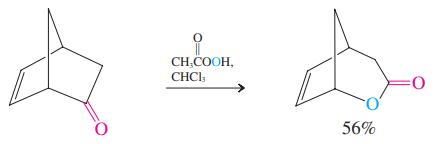
Transcribed Image Text:
CH,COOH, CHCI, 56%
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Solution Mechanism CH3COOH CHCl3 CH3C00H H Minor ...View the full answer

Answered By

Mohd Rehan
I teach in a local coaching centre for 4 years in Aligarh India. I am pursuing PhD from AMU Aligarh. I am all India qualified tests NET (National Eligibility Test) and GATE. Four certificates from NPTEL (1) Medicinal chemistry from IISER Pune, (2) Retrosynthesis from IIT Kharagpur (3) Application of NMR from IISc Bangalore, (4) Multidimensional NMR from IISc Bangalore. Eight research articles have been published in the chemistry field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore
Question Posted:
Students also viewed these Sciences questions
-
Formulate a detailed mechanism for the Baeyer-Villiger oxidation of the ketone shown in the margin. %3=
-
Formulate a detailed mechanism for the Mannich reaction between 2-methylpropanal, formaldehyde, and methanamine shown on page 994. Page 994 Mannich Reaction CH,N(CH,), Cl- 1. H HCI, CH,CH,OH, A +...
-
A synthesis of the b-receptor blocker called toliprolol begins with a reaction between 3-methylphenol and epichlorohydrin. The synthesis is outlined below. Give the structures of the intermediates...
-
DRAW A PLAN OF YOUR HOME THEN ESTIMATE QUANTITY OF * FOOTING *BLOCK WALL UNDER DPC * BLOCK WALL ABOVE DPC * CEMENT PLASTERING OUTSIDE *GYPSUM PLASTERING INSIDE
-
Suppose that a newspaper commentator calls for a quick devaluation of the U.S. dollar as a temporary boost to the U.S. economy in order to get it out of a slump. Could you endorse such a suggestion?...
-
Explain the major challenges in hedge fund activism research.
-
Quilts R Us (QRU) is considering investing in a new patterning attachment with the cash flow profile shown in the table below. QRU's MARR is 13.5 percent/year. a. What is the internal rate of return...
-
Digital Controls, Inc. (DCI) manufactures two models of a radar gun used by police to monitor the speed of automobiles. Model A has an accuracy of plus or minus 1 mile per hour, whereas the smaller...
-
We read about Language and Authority in grammar and how some groups try to control language. Identify one such group and explain how and why they try to control language. Do you think such efforts to...
-
During the year, Angela and Michael reported the $120,000 of gross earnings. Angela is a self-employed CPA and Michael is a tax manager employed at local CPA firm. Angela incurred normal...
-
Formulate in full detail the mechanism for the Wolff-Kishner reduction of 1-phenylethanone (acetophenone) to ethylbenzene (see p. 803). Data from page 803. CH3 CH3 1. H;NNH, H:O, diethylene glycol,...
-
Give the two theoretically possible Baeyer-Villiger products from each of the following compounds. Indicate which one is formed preferentially. (a) (b) (c) (d) (e) C. CH3 H.
-
Solve the systems in Problems 27-54 by solving the corresponding matrix equation with an inverse, if possible. \(\left\{\begin{array}{l}2 x+3 y=-22 \\ x-6 y=49\end{array}ight.\)
-
Real-World Case 21.2 Monica's best friend Shanna lives out of state and her mom just had a breast biopsy. Shanna calls Monica upset because they have not received the results of the biopsy. Shanna...
-
Consider the following graph: a d b ge h 1. Can the nodes of the graph be partitioned to create a Bi-Partite Graph? Why or why not? 2. Give a sequence of nodes that comprises and Euler circuit...
-
Does Texas require a special license for the distribution of alcoholic beverages at private events? Does Texas state law forbid distribution of alcohol to those attending a private event who are...
-
one of my favorite motivational theories is the Motivation-Hygiene Theory developed by Frederick Herzberg. The theory assesses intrinsic and extrinsic factors as well as job satisfaction, and states...
-
Write down the Difference and Comparison of Indian Contract Law and Australian contract law Analyse and recommendations ?
-
Asok's AGI for 2014 is $133,050. Included in this AGI is a $45,000 25% long-term capital gain and a $13,000 0%/15%/20% long-term capital gain. Asok is single, uses the standard deduction, and has...
-
A company pledges their receivables so they may Multiple Choice Charge a factoring fee. Increase sales. Recognize a sale. Collect a pledge fee. Borrow money. Failure by a promissory notes' maker to...
-
Explain whether each pair of models represents isomers or the same compound. (All represent compounds with the formula C7H16.) Draw structures for each compound represented by the models.
-
Explain whether each pair of models represent isomers or the same compound. Draw structures for each compound represented by the models.
-
The following models represent three isomers of C6H4Cl2. Explain which of these compounds does not have a dipole moment.
-
Silverton Confectionery is a growing Berkshire-based company specialising in selling quality chocolates and sweets at higher than average prices through newsagents and confectioners. At present their...
-
4. X, the proprietor of a departmental store, decided to calculate separate profits for his two departments L and M for the month ending 31st January. Stock on 31st January could not be valued for...
-
What level of confidentiality should be attached to the preparation and handling of a memorandum of law? Why? Assume you have been working for a legal specialist in estate law for a number of years...

Study smarter with the SolutionInn App


