The selectivity of hydroboration increases with increasing bulkiness of the borane reagent. (a) For example, 1-pentene is
Question:
The selectivity of hydroboration increases with increasing bulkiness of the borane reagent.
(a) For example, 1-pentene is selectively hydroborated in the presence of cis-and trans-2-pentene when treated with bis(1,2-dimethylpropyl)borane (disiamylborane) or with 9-borabicyclo[3.3.1]- nonane, 9-BBN. Divide the task of formulating the structure of the starting alkene used in preparing both of these bulky borane reagents among yourselves. Make models to visualize the features of these reagents that direct the structural selectivity.
(b) In an enantioselective approach to making secondary alcohols, two equivalents of one enantiomer of a-pinene are treated with BH3. The resulting borane reagent is treated with cis-2-butene followed by basic hydrogen peroxide to yield optically active 2-butanol.
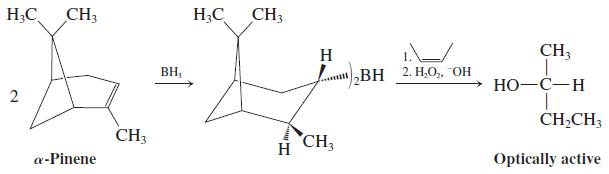
Share your model kits to make a model of a-pinene and the resulting borane reagent. Discuss what is directing the enantioselectivity of this hydroboration – oxidation reaction. What products besides 2-butanol result from the oxidation step?
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





