This problem introduces two literature syntheses of indole derivatives, and you are asked to come up with
Question:
This problem introduces two literature syntheses of indole derivatives, and you are asked to come up with plausible mechanisms for them. Divide your team in two, each group concentrating on one of the methods.
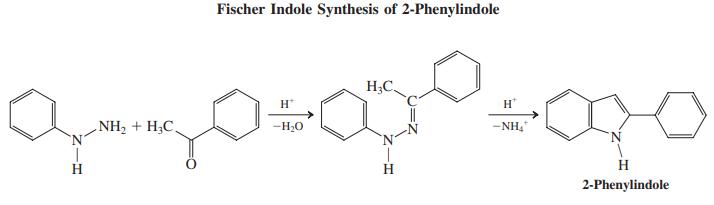
In this procedure, a hydrazone of an enolizable aldehyde or ketone is heated in strong acid, causing ring closure with simultaneous expulsion of ammonia to furnish the indole nucleus. [The mechanism of the reaction proceeds in three stages: (1) an imine – enamine tautomerization); (2) an electrocyclic reaction (a “diaza-Cope” rearrangement;); (3) another imine – enamine (in this case, benzenamine) tautomerization; (4) ring closure to the heterocycle; and (5) elimination of NH3.]
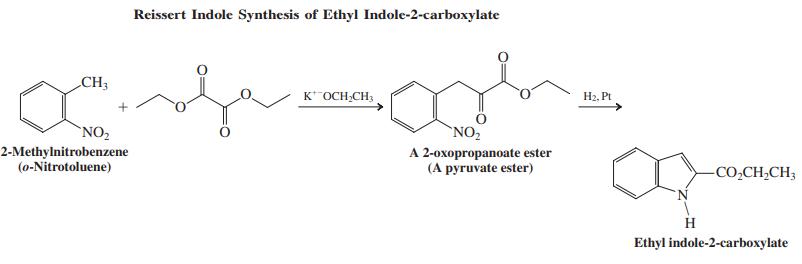
In this sequence, a 2-methylnitrobenzene (o-nitrotoluene) is first converted into an ethyl 2-oxopropanoate ester, which, on reduction, is transformed into the target indole.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





