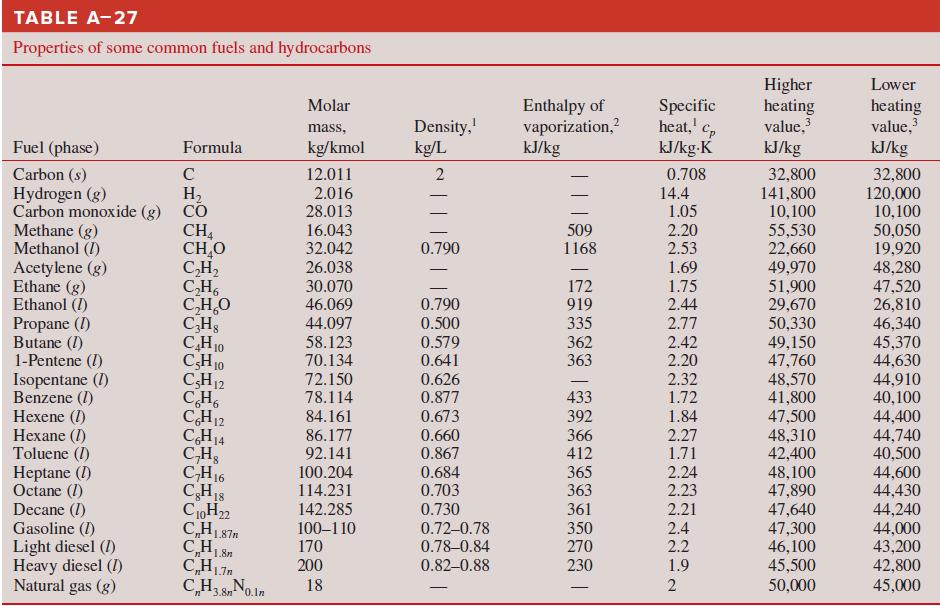
Calculate the HHV and LHV of gaseous n-octane fuel (C 8 H 18 ). Compare your results
Question:
Calculate the HHV and LHV of gaseous n-octane fuel (C8H18). Compare your results with the values in Table A–27.
Transcribed Image Text:
TABLE A-27 Properties of some common fuels and hydrocarbons Higher heating value, Lower Enthalpy of vaporization,? kJ/kg Specific heat,' kJ/kg-K Molar Density, kg/L heating value, kJ/kg 2 mass, Fuel (phase) Formula kg/kmol kJ/kg Carbon (s) Hydrogen (g) Carbon monoxide (g) CO Methane (g) Methanol (I) Acetylene (g) Ethane (g) Ethanol (I) Propane (I) Butane (I) 1-Pentene (I) Isopentane (1) Benzene (1) Hexene (1) Hexane (I) Toluene (I) Heptane (I) Octane (1) Decane (I) Gasoline (I) Light diesel (I) Heavy diesel (I) Natural gas (g) 32,800 141,800 10,100 55,530 22,660 49,970 51,900 29,670 50,330 49,150 47,760 48,570 41,800 C 12.011 0.708 32,800 120,000 10,100 50,050 19,920 48,280 47,520 26,810 46,340 45,370 44,630 44,910 40,100 H, 2.016 28.013 14.4 1.05 CH, CH,O C,H, C,H, C,H,O CHs CH10 CH10 CH12 CH, CH12 CH14 C,H, CH16 CH18 C0H22 C,H187m 16.043 32.042 26.038 509 1168 2.20 0.790 2.53 1.69 30.070 46.069 172 919 1.75 0.790 2.44 335 362 363 44.097 0.500 2.77 58.123 70.134 72.150 0.579 0.641 2.42 2.20 2.32 0.626 0.877 433 392 366 412 78.114 1.72 84.161 0.673 1.84 47,500 48,310 42,400 48,100 47,890 47,640 47,300 46,100 45,500 44,400 44,740 40,500 44,600 44,430 44,240 44,000 43,200 42,800 86.177 0.660 2.27 1.71 2.24 92.141 0.867 100.204 114.231 0.684 0.703 365 363 2.23 142.285 0.730 361 2.21 100–110 170 0.72-0.78 0.78-0.84 350 270 2.4 2.2 200 0.82–0.88 230 1.9 CH3 8No.ln C,H3,8n0.ln 50,000 45,000 18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The higher and lower heating values of gaseous octane are to be determined and compared to the liste...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Engineering questions
-
Calculate the HHV and LHV of liquid propane fuel (C3H8). Compare your results with the values in Table A-27 109258336914756 4202 367073567 88156996317585341863150 21052919097817828777650...
-
Calculate the higher and lower heating values of gaseous methane fuel (CH 4 ). Compare your results with the values in Table A27.
-
Compare your results with those obtained in Problem 8.113 (p. 330)? Microbiology Refer to the data in Table 8.28 (p. 330). Table 8.28 Pod weight (g) from inoculated (I) and uninoculated (U) plantsa
-
Write the nodal equations for the networks in Figure. Using determinants, solve for the nodal vo ltages. R4 2 5 A R 1 R3 50 4 3 A
-
A student experiences difficulties with malfunctioning alarm clocks. Instead of using one alarm clock, he decides to use three. What is the probability that at least one alarm clock works correctly...
-
Evaluate the given expressions, the numbers are approximate. 346.4 - 23.5 287.7 0.9443 (3.46)(0.109)
-
Why is the concept of an ongoing concern important to the interpretation of financial statements?
-
Casey Carpet manufactures broadloom carpet in seven processes: spinning, dyeing, plying, spooling, tufting, latexing, and shearing. In the Dyeing Department, direct materials (dye) are added at the...
-
Many colleges and universities are supported by the government, while others are supported by private organizations. What bearing does the source of support have on the determination of authoritative...
-
Bachinos Pizzas accounts follow. The company has just completed its first year of operations ended September 30, 2014. Accounts Payable.......... $ 21,000 Accounts Receivable........... 26,400...
-
Repeat Prob. 1546 for liquid octane (C 8 H 18 ). Data From Q#46: Determine the enthalpy of combustion of methane (CH 4 ) at 25C and 1 atm, using the enthalpy of formation data from Table A26. Assume...
-
Propane fuel (C 3 H 8 ) is burned in a space heater with 50 percent excess air. The fuel and air enter this heater steadily at 1 atm and 17C, while the combustion products leave at 1 atm and 97C....
-
Find the first partial derivatives of the function. h(x, y, z, t) = x 2 y cos(z/t)
-
The responses to which questions lead to determining decision context? Select answer from the options below How fast and what format is the information needed? Who will use it, and why is the...
-
They are planning to take on Canada again - they will have to play better. What do we call this type of growth?
-
The effective management of time is crucial for students success. What is the importance of effective time management to successful study? Explain your answers and reasoning. You must discuss and...
-
How do you tend to think about percent? Do you break down the word: per cent to per hundred? Do you think of it as a ratio: 1/100? Do you think of it as a decimal? Do you have any shortcuts for...
-
An engineer prepares an estimate to provide structural engineering services for a new housing facility. The total construction budget for the office complex is $120 million dollars. The design team...
-
Is it possible for the auditor to conduct the audit without reliance on management? Why or why not?
-
Banner Company acquires an 80% interest in Roller Company for $640,000 cash on January 1, 2013. The NCI has a fair value of $160,000. Any excess of cost over book value is attributed to goodwill. To...
-
Determine the highest possible temperature that can be obtained when liquid gasoline (assumed C8H18) at 25oC is burned steadily with air at 25oC and 1 atm. What would your answer be if pure oxygen at...
-
Liquid propane (C3H8()) enters a combustion chamber at 25oC and 1 atm at a rate of 0.4 kg/min where it is mixed and burned with 150 percent excess air that enters the combustion chamber at 25oC. The...
-
Determine the work potential of 1 lbmol of diesel fuel (C12H26) at 77oF and 1 atm in an environment at the same state.
-
The following balance sheet for the Los Gatos Corporation was prepared by a recently hired accountant. In reviewing the statement you notice several errors. LOS GATOS CORPORATION Balance Sheet At...
-
Using Regression to Calculate Fixed Cost, Calculate the Variable Rate, Construct a Cost Formula, and Determine Budgeted Cost Pizza Vesuvio makes specialty pizzas. Data for the past 8 months were...
-
Mackenzie Corp. is preparing the December 31, 2023, year-end financial statements. Following are selected unadjusted account balances: Estimated warranty liability $ 6,650 Income tax expense Mortgage...

Study smarter with the SolutionInn App


