Estimate the c p of nitrogen at 300 kPa and 400 K, using (a) The relation in
Question:
Estimate the cp of nitrogen at 300 kPa and 400 K, using
(a) The relation in Prob. 12–91,
Data From Q#91:
Consider an infinitesimal reversible adiabatic compression or expansion process. By taking s = s(P, v) and using the Maxwell relations, show that for this process Pvk = constant, where k is the isentropic expansion exponent defined as
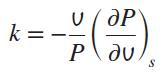
Also, show that the isentropic expansion exponent k reduces to the specific heat ratio cp/cv for an ideal gas.
(b) Its definition. Compare your results to the value listed in Table A–2b.
Transcribed Image Text:
U ( OP k = - P du
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The c p of nitrogen at 300 kPa and 400 K is to be estimated using the relation given and its defini...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
Consider an infinitesimal reversible adiabatic compression or expansion process. By taking s = s(P, v) and using the Maxwell relations, show that for this process Pvk = constant, where k is the...
-
Consider an infinitesimal reversible adiabatic compression or expansion process. By taking s = s(P, v) and using the Maxwell relations, show that for this process Pvk = constant, where k is...
-
Estimate the cp of nitrogen at 300 kPa and 400 K, using (a) The relation in the above problem and (b) Its definition. Compare your results to the value listed in Table A2b.
-
Show that for an integer n > 2, the period of the decimal expression for the rational number is at most n - 1. Find the first few values of n for which the period of - is equal ton- 1. Do you notice...
-
The accompanying table lists weights (in kilograms) of plastic discarded by a sample of households, along with the sizes of the households (adapted from Data Set 1). Is there a significant linear...
-
Shay Kinderton is a new payroll accountant with Sals Diamond Mart, Inc., in New York, NY. The company had a payroll tax liability of $49,580 during the most recent lookback period. For the quarter...
-
Consider the $4 \mathrm{x} 4$ square matrix below. \[\left[\begin{array}{llll}A & B & C & D \\D & A & B & C \\C & D & A & B \\B & C & D & A\end{array} ight]\] Suppose the rows correspond to subjects...
-
Suppose you manage Outward Bound, Inc., a Vermont sporting goods store that lost money during the past year. To turn the business around, you must analyze the company and industry data for the...
-
How do strategic leaders leverage data-driven insights and emerging technologies to anticipate market trends and capitalize on strategic opportunities in a rapidly evolving landscape?
-
Richard chooses technique 0 and 2 requiring 10+10-20 efforts and provising 10+11=21 benefits. Hence, 21 is returned as the output Example 2: input1: 3 input2: (10,10,10,10) input3: (10,11,12,15)...
-
For ideal gases, the development of the constant-volume specific heat yields Prove this by using the definitions of pressure and temperature, T = (u/s)v and P = (u/v)s. = 0 du.
-
Propane at 500 kPa and 100C is compressed in a steady-flow device to 4000 kPa and 500C. Calculate the change in the specific entropy of the propane and the specific work required for this compression...
-
How do you define a completion, and how does this definition help a researcher deal with incomplete questionnaires?
-
In March of 2016, Alphabet (the parent of Google) announced they plan to sell their Boston Dynamics business. Boston Dynamics builds high end legged robots). Alphabet's executives said they wanted...
-
What is your interest rate? Credit Score Interest Chart Credit Score Average APR (New car) Average APR (Used car) Superprime: 781-850 2.40% 3.71% Prime: 661-780 3.56% 5.58% Nonprime: 601-660 6.70%...
-
The United States has the world's largest and strongest economy, like... ever in the history of humankind. How can one have resource scarcity in its current setting? Is it perhaps because people find...
-
Which model proposes that, while a country pursues economic development, income inequality will grow during, but once the country is adequately "developed" or "industrialized" economic inequality...
-
Washington D.C. implements a policy beginning in 2037 requiring firms to provide paid child leave. You want to study the effect of this policy on firm employment and average wages. You know that...
-
Refer to the preceding problem. a. Will Carl owe interest? If so, on what amount and for how many days? b. Assume the applicable interest rate is 6%. Compute Carl's interest payable if his 2013 tax...
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
A Heat pump water heater (HPWH) heats water by absorbing heat from the ambient air and transferring it to water. The heat pump has a COP of 3.4 and consumes 6 kW of electricity when running....
-
A heat pump that operates on the ideal vapor-compression cycle with refrigerant-134a is used to heat a house. The mass flow rate of the refrigerant is 0.25 kg/s. The condenser and evaporator...
-
A large refrigeration plant is to be maintained at - 15oC, and it requires refrigeration at a rate of 100 kW. The condenser of the plant is to be cooled by liquid water, which experiences a...
-
Reflecting on the book Who Gets Promoted, Who Doesn't, and Why (Asher, 2008), answer the following questions in depth. Explain the statement, "all business is sales." What six things matter? If...
-
Reflecting on the book Who Gets Promoted, Who Doesn't, and Why (Asher, 2008), answer the following questions in depth. No matter what you have done in the past, the boss really doesn't care. Why?...
-
Do you think Richard Scrushy should have been found guilty or acquitted for his role in the case against HealthSouth? https://www.wsj.com/articles/SB111702610398942860

Study smarter with the SolutionInn App


